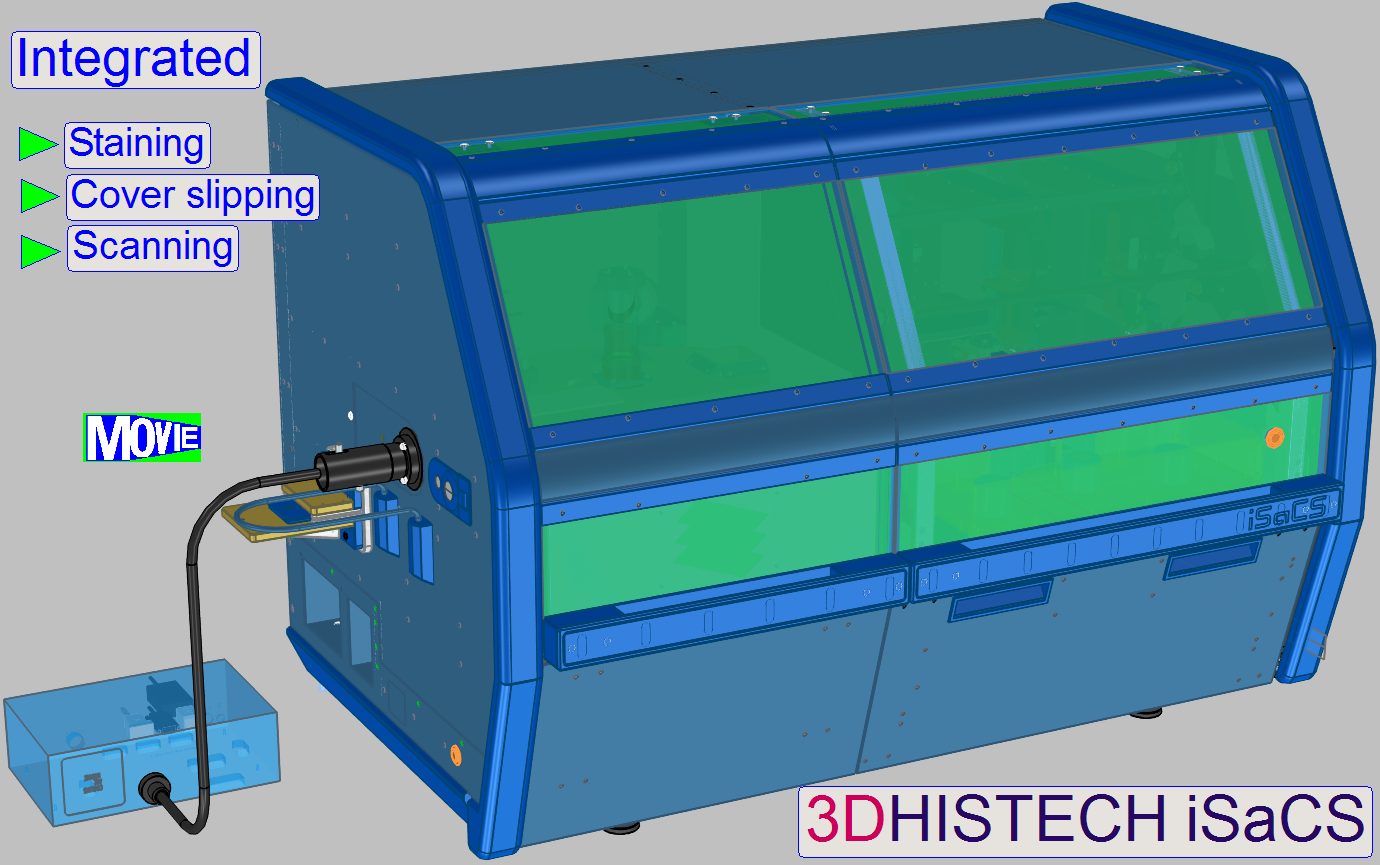
Overview; iSaCS
For
technicians and sales managers
This section describes the tasks, units and components
of the 3DHISTECH Immunohistochemistry Staining automatic Cover slipping and Scanning
equipment (iSaCS).
Features
· Complex and integrated
solution with small footprint
· Handling of 72
slides simultaneously
· The modular
construction allows to execute different tasks concurrently
· Immunohistochemistry
and counterstaining of tissue specimen
· Application of
stain during staining procedure
· Application of
mounting medium during cover slipping
· Automatic scan of
the created specimen in fluorescent (FL) or brightfield (BF) scan mode
· Automatic scan
session performed in 30-120 seconds in brightfield scan mode for 1 specimen
Capacities
·
72 slides with specimen
·
150 cover slips
·
15 ml cover slip mounting medium (for 300 slides)
·
40 reagent vials
·
8 ml vial (for
80 staining procedures)
·
1 x 5l tank for washing fluid (for 200 slides)
·
1 x 5l tank for rinsing fluid (for 200 slides)
·
2 x 5l tanks for waste fluid (for 200 slides)
Main part list
|
Component |
Stepper motor |
Actuator |
Sensor |
Light source |
Camera |
|
|
STA |
Staining module |
4 |
0 |
2 |
0 |
0 |
|
COV |
Cover slipper |
3 |
2 |
3 |
0 |
0 |
|
TRA |
Transporter |
3 |
1 |
1 |
0 |
0 |
|
WRU |
Wash - rinse unit |
4 |
2 |
12 |
0 |
0 |
|
REA |
Reagent changer |
1 |
0 |
0 |
1 |
1 |
|
SLI |
Slide image |
0 |
0 |
0 |
2 |
1 |
|
AIR |
Air wiper |
0 |
1 |
0 |
0 |
0 |
|
FRH |
Frame; housing |
0 |
0 |
4 |
1 |
0 |
|
|
Digital scanner |
6 |
0 |
2 |
3 |
2 |
|
Total |
iSaCS |
21 |
6 |
24 |
7 |
4 |
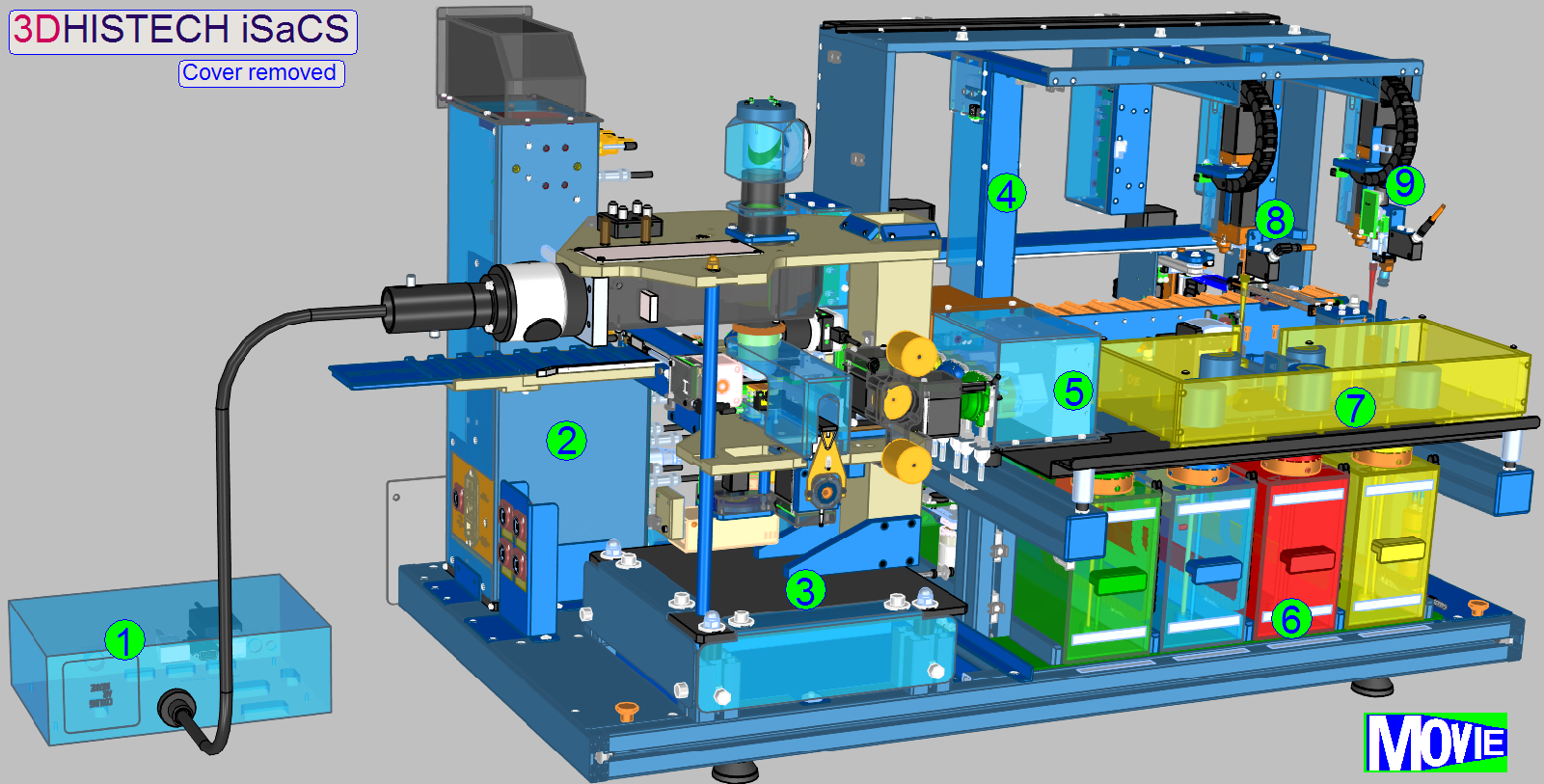 The iSaCS is a complex, small footprint,
integrated solution for the automation of tissue antigen marking. It is
intended to perform IHC (Immunohistochemistry) staining, FISH (Fluorescent In
Situ Hybridization) staining, counterstaining, cover slipping and scanning. The
iSaCS was designed to use a modular construction approach, with the aim of
having numerous modules working simultaneously.
The iSaCS is a complex, small footprint,
integrated solution for the automation of tissue antigen marking. It is
intended to perform IHC (Immunohistochemistry) staining, FISH (Fluorescent In
Situ Hybridization) staining, counterstaining, cover slipping and scanning. The
iSaCS was designed to use a modular construction approach, with the aim of
having numerous modules working simultaneously.
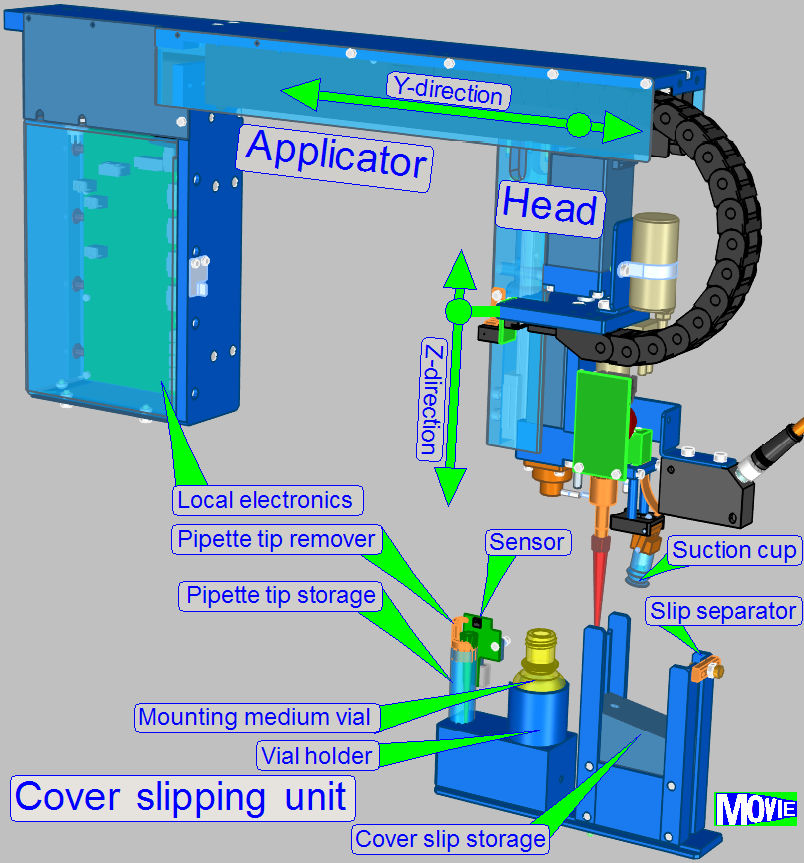
- Lumencor SPECTRA
- Power tower
- Slide
scanner
- Specimen
image
- Peristaltic pumps
- Fluid containers
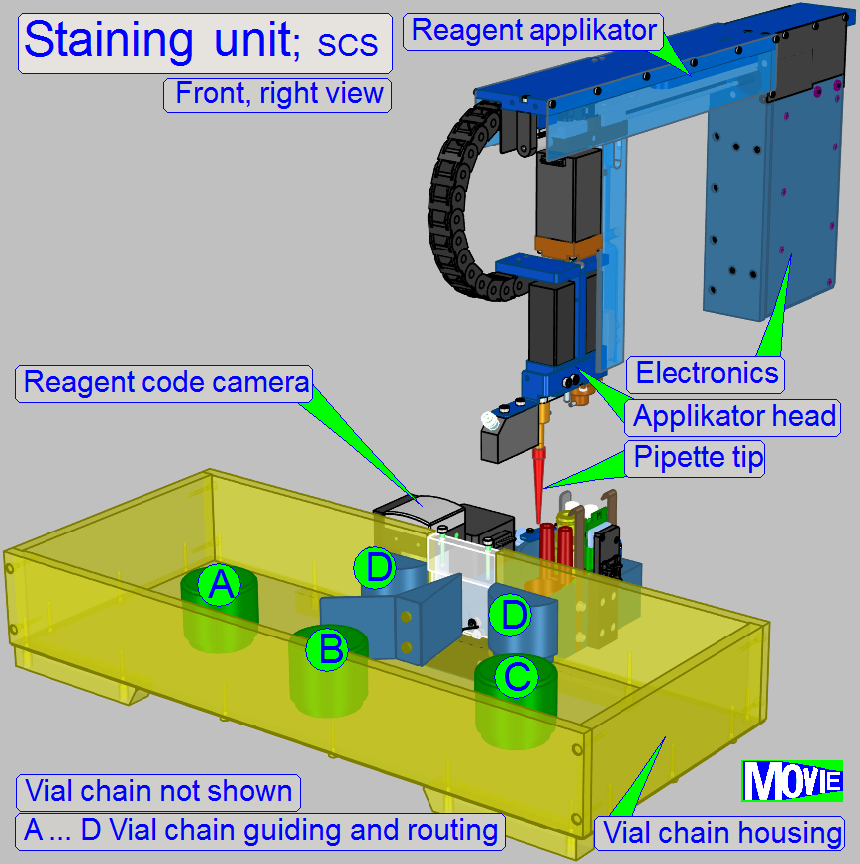
- Reagent
changer
- Reagent
applicator
- Cover slipping unit
The Lumencor SPECTRA Light Engine is an external light source that
illuminates the field of view at fluorescent scanning procedure. It is connected
to the iSaCS through the left side service door of the equipment with a light
cable. The Lumencor has its own power cable through which it receives the
necessary power.
![]() Lumencor SPECTRA light
engine®
Lumencor SPECTRA light
engine®
Power
tower
The main electronics of the iSaCS are stored in the power tower. It is
located behind the scanner module in the equipment.
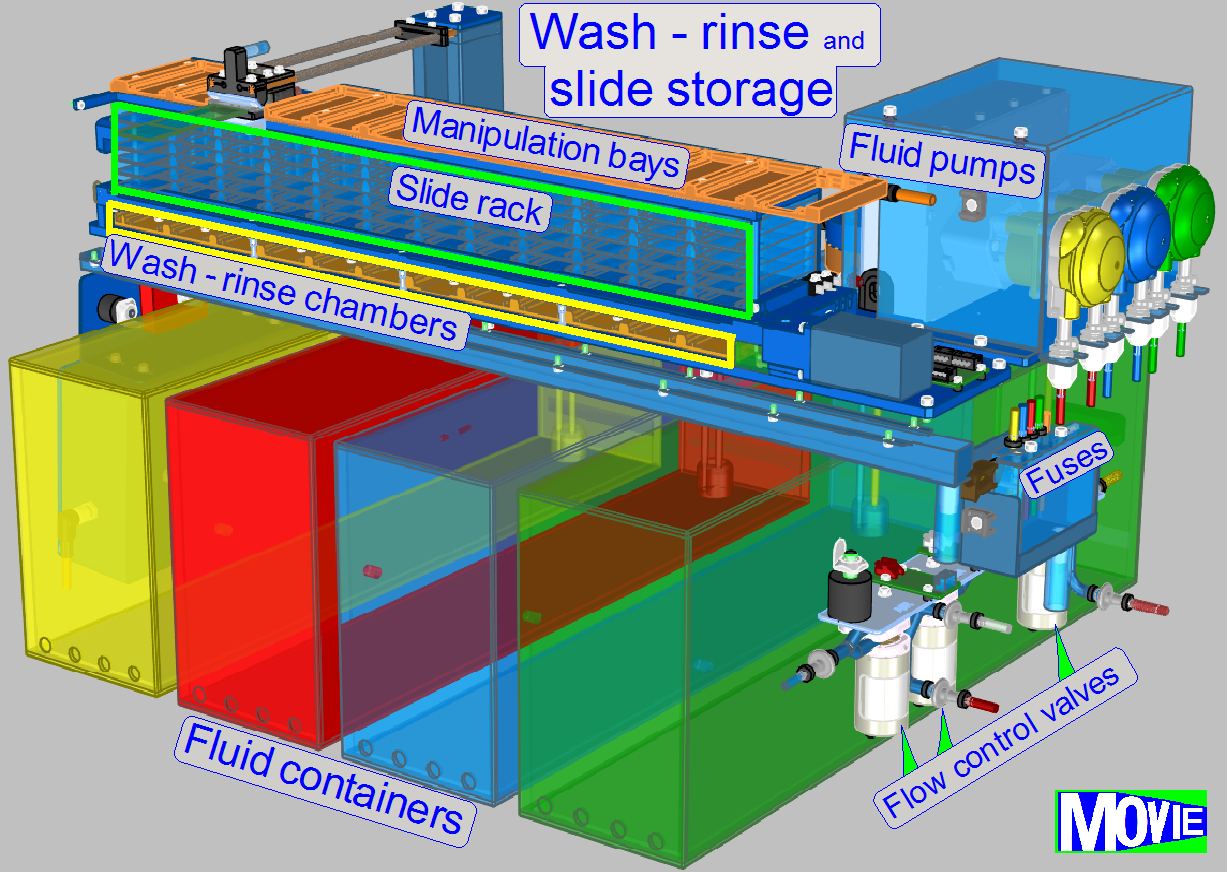
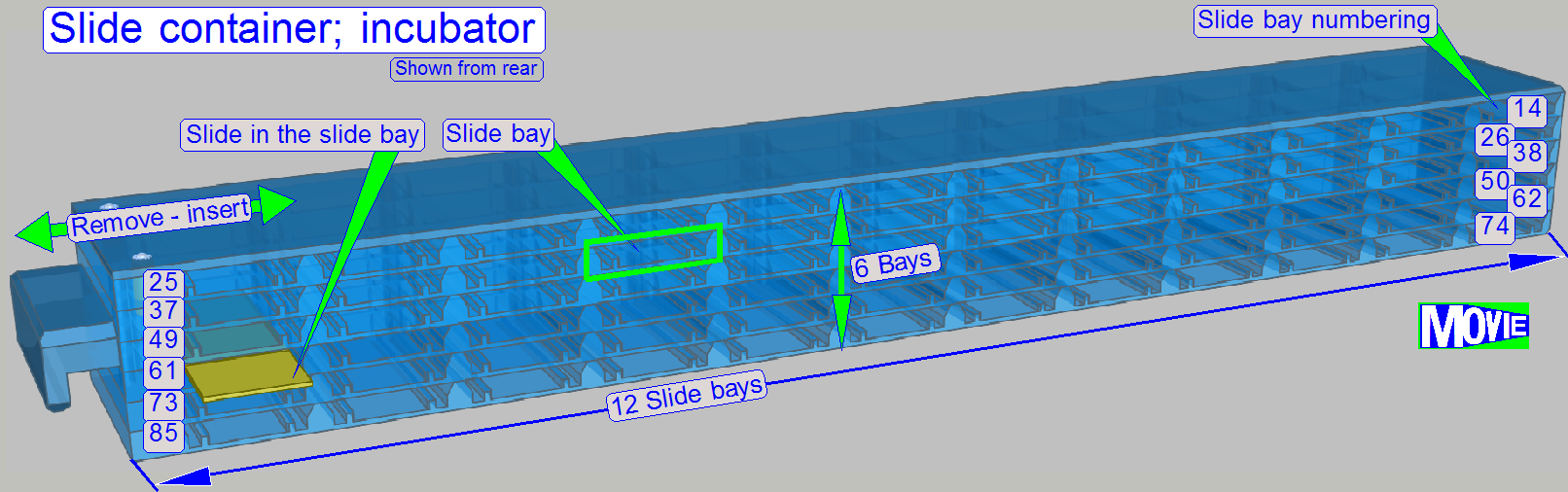
Slide storage
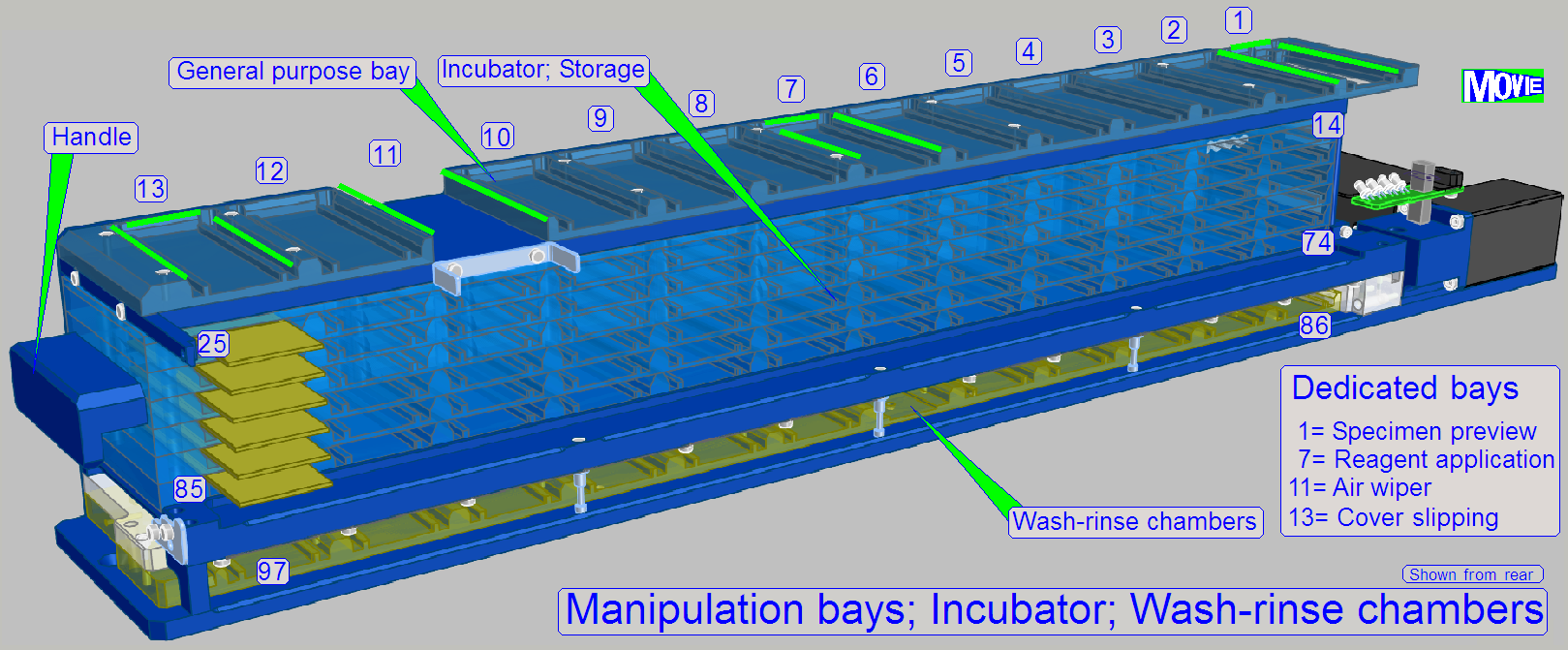 The user fills the
slide rack (incubator) with slides containing the specimen.
The user fills the
slide rack (incubator) with slides containing the specimen.
For this purpose, 72 (12x6) slide bays are arranged in the rack.
To fulfill the tasks, dedicated slide bays are defined; always in these
positions the named task will be executed; general purpose bays are used e.g.
for sorting tasks.
· 1= Specimen Image; barcode capturing
· 7= Reagent application
· 11= Air wiper
· 13= Cover slipping
12
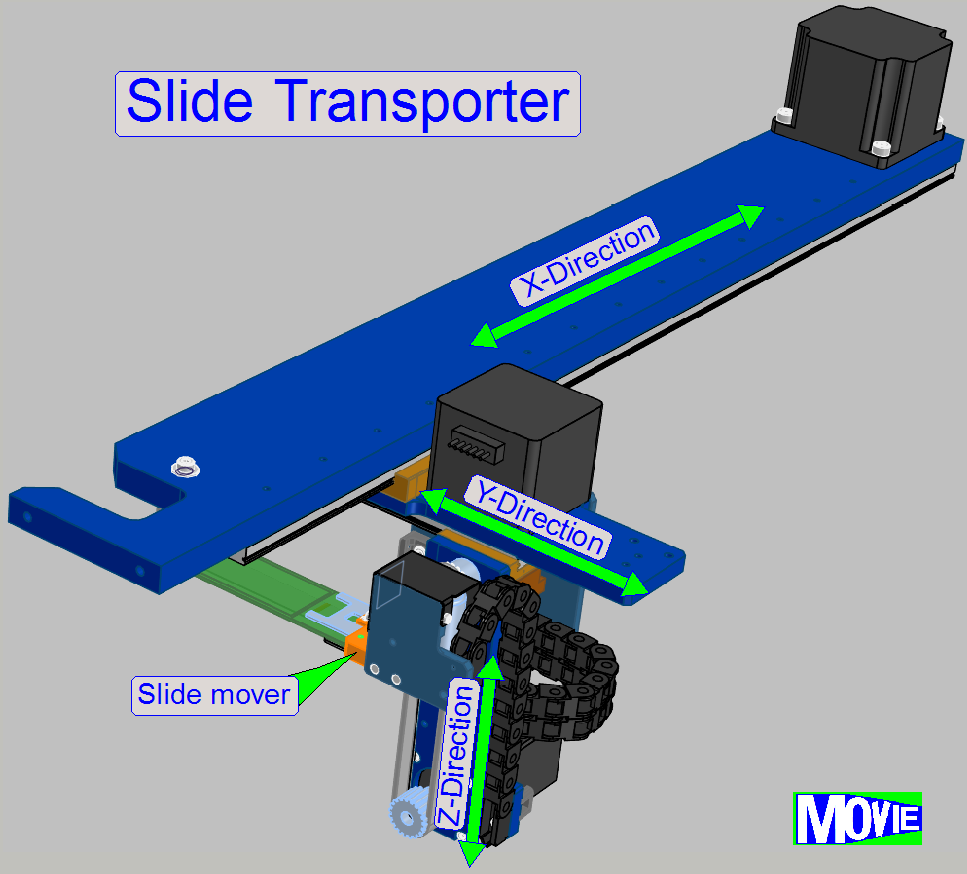
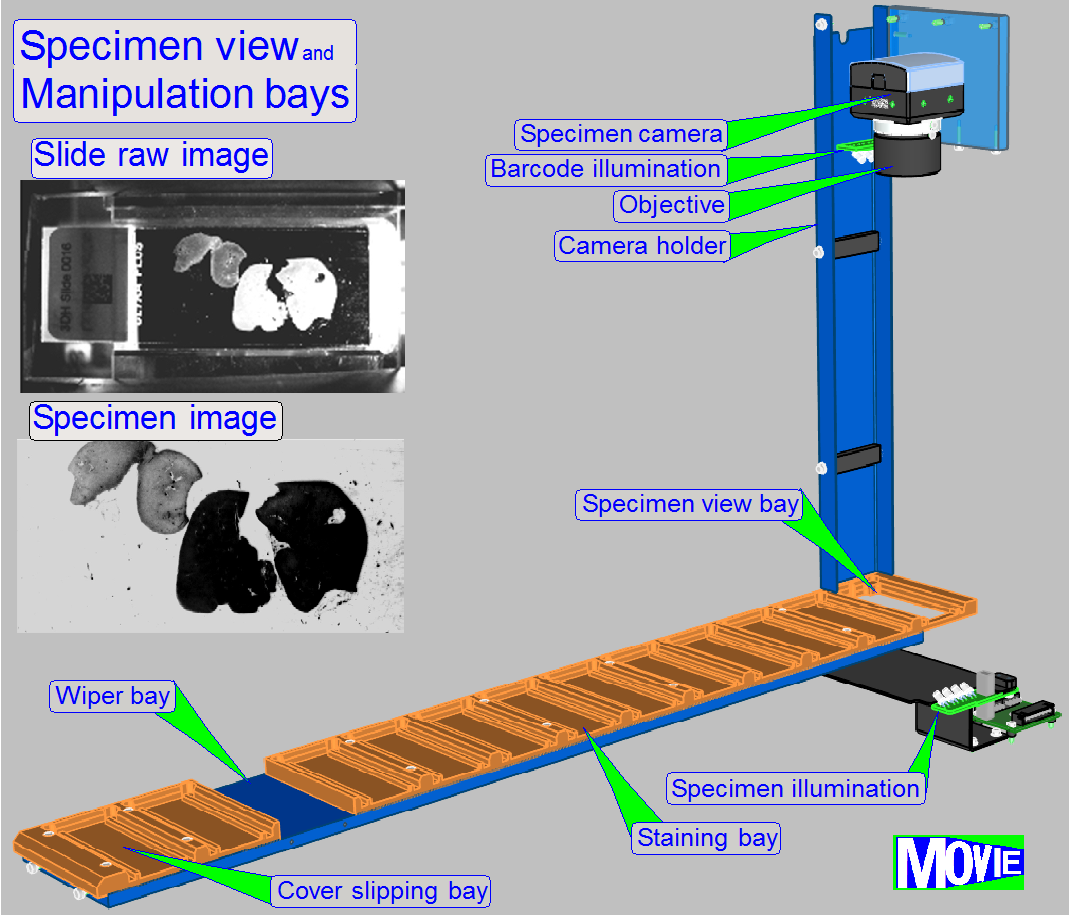
·
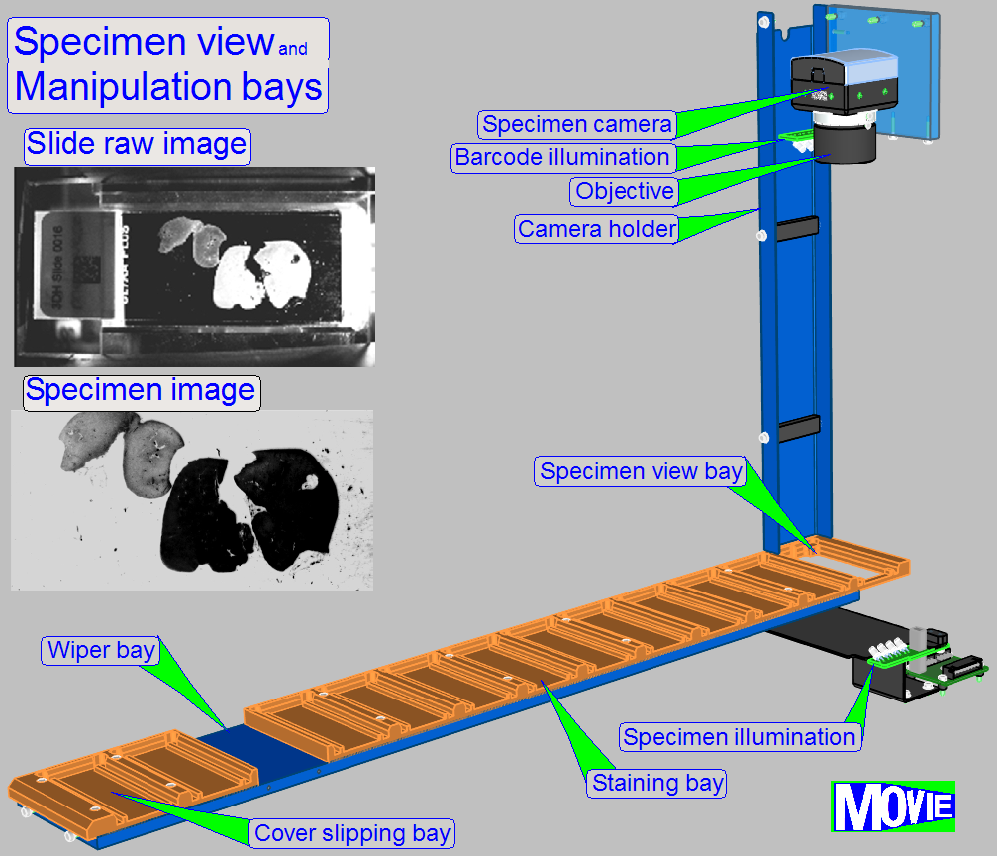 localization of tissue specimen on the slide
localization of tissue specimen on the slide
·
slide identification
through DataMatrix barcode label
· special
illumination for tissue specimen with low contrast
·
fast positioning (approx. 5 s) of slides anywhere inside the slide
storage and staining-cover-slipping unit
· dedicated motion
schemes for different movement types
The transporter moves the slide from the slide rack position to
- preview unit
- staining unit
- wash and
rinse chambers
- Air wiper
position
- cover
slipping unit
- any possible
position
- finally to
the slide scanner and return to its origin position
 To determine the quantity of
required liquid exactly, an “acoustic fluid level detector” is implemented. The
distance of the liquid surface to the pipette tip has to be known. If the
distance is known exactly, the immersion depth of the pipette to gather the
required liquid quantity for the process can be calculated, because the
diameter of the vials is known.
To determine the quantity of
required liquid exactly, an “acoustic fluid level detector” is implemented. The
distance of the liquid surface to the pipette tip has to be known. If the
distance is known exactly, the immersion depth of the pipette to gather the
required liquid quantity for the process can be calculated, because the
diameter of the vials is known.
· The distance of
the liquid surface to the pipette tip is determined acoustically.
It is important to
gather the required quantity of the appropriate liquid very precise. At the end of the fluid application process,
the pipette has to be empty! If the quantity of fluid in the pipette is too
less, the process result becomes poor, otherwise, if fluid remains in the
pipette, the level in the pipette will increase more and more and a waste of
(often very expensive) fluid occurs. Furthermore, if the lfluid will dry in the
pipette, waste process results are produced.
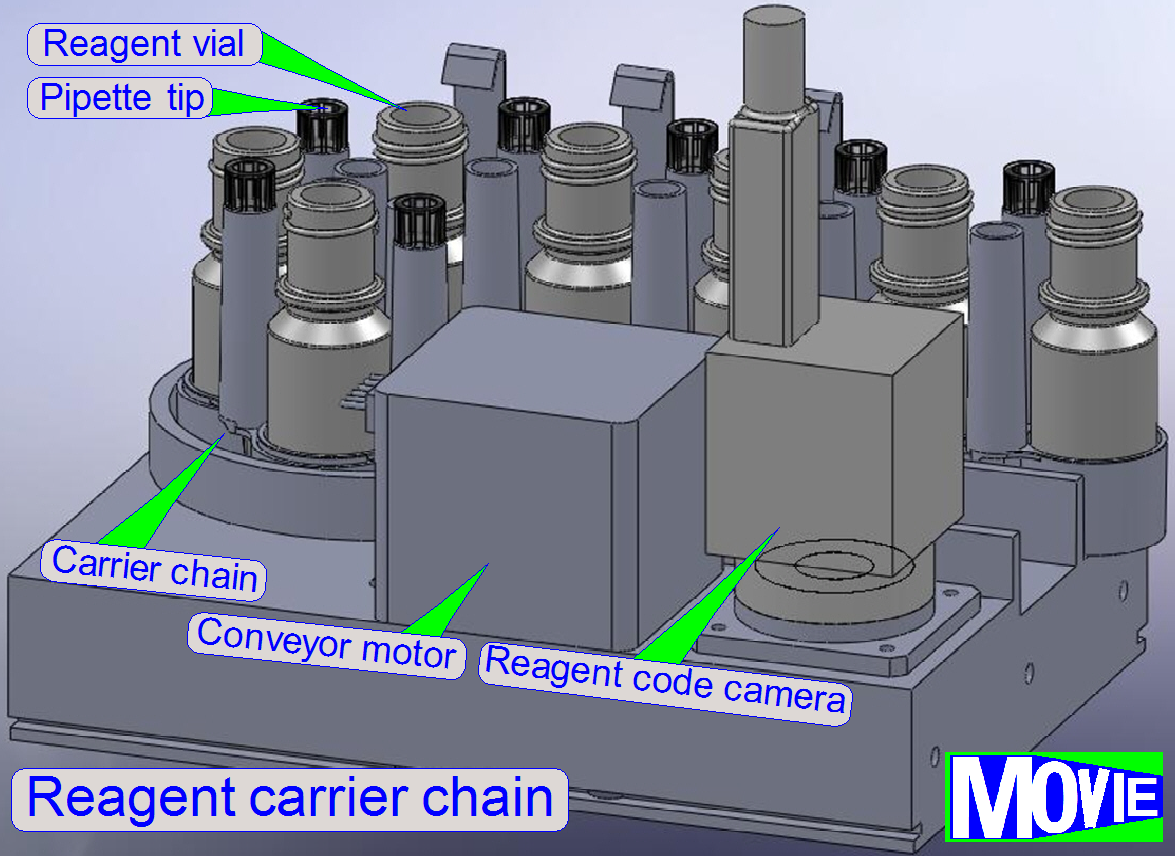
·
freely variable number (up to 40
pcs.) of 8 ml reagent vials
· vial identification
through barcode Data Matrix labels
·
disposable
pipette tips (200 μl) dedicated to each reagent
·
reagent
level measurement with 100 μl precision
·
reagent
dispensing along any arbitrary trajectory
· precise covering of the specimen surface
exclusively, using the lowest possible amount of reagent
·
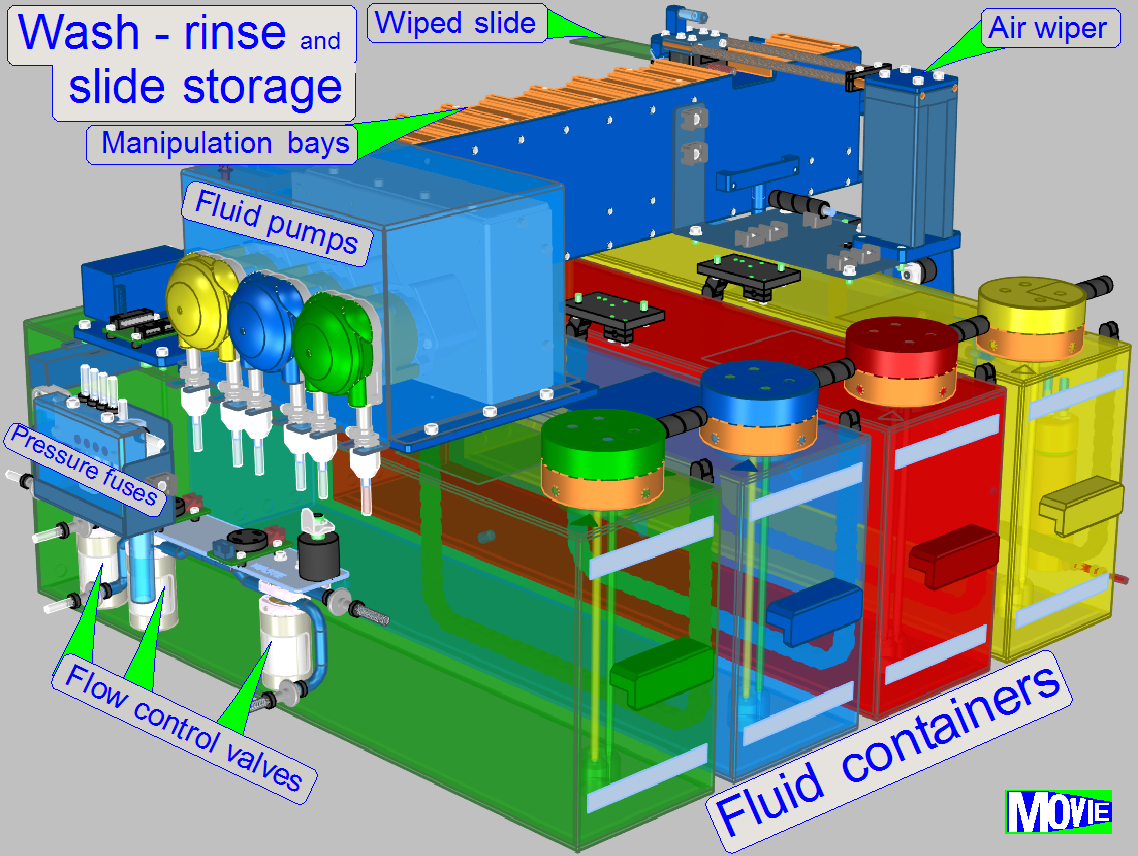
Washing and rinsing is done by the help of dedicated peristaltic pumps
for distilled water, wash buffer and fluid waste.
· bubble detection
in the tubing, priming of pumps with opto-tube sensors
·
simultaneous
use of multiple wash-and-rinse chambers possible, capacity: 12 slides
·
precisely
controlled flooding and draining of chambers, wash-and-rinse volume: 1-3 ml
·
residual fluid amount on slide surface: less than 10 μl
·
small volume of
single-slide incubation chambers minimizes evaporation
· incubation
chambers in compact, small footprint arrangement, capacity: 72 slides
·
control
of relative humidity and temperature
·
Conventional glass
cover slips of different sizes, capacity: 200
pcs.
· Verification of
the number of available cover slips with +/- 1 pc. precision
·
separation
of multiple adhered cover slips
·
aqueous
mounting medium, capacity: 10 ml
·
disposable
pipette tip (200 μl) for mounting medium
· mounting medium level measurement
with 100 μl precision
·
pick-and-place
cover slipping procedure using suction cup
·
pressure
monitoring in the vacuum system
·
controlled and precise placement of cover-slip onto
the sample
Workflow
The sample to be stained is placed on a glass
slide together with its barcode and this will be inserted into the iSaCS.
Allowed slide dimensions are defined as:
Length: 75.00
to 76.00 mm
Width: 25.00
to 26.00 mm
Thickness: 00.95
to 01.05 mm
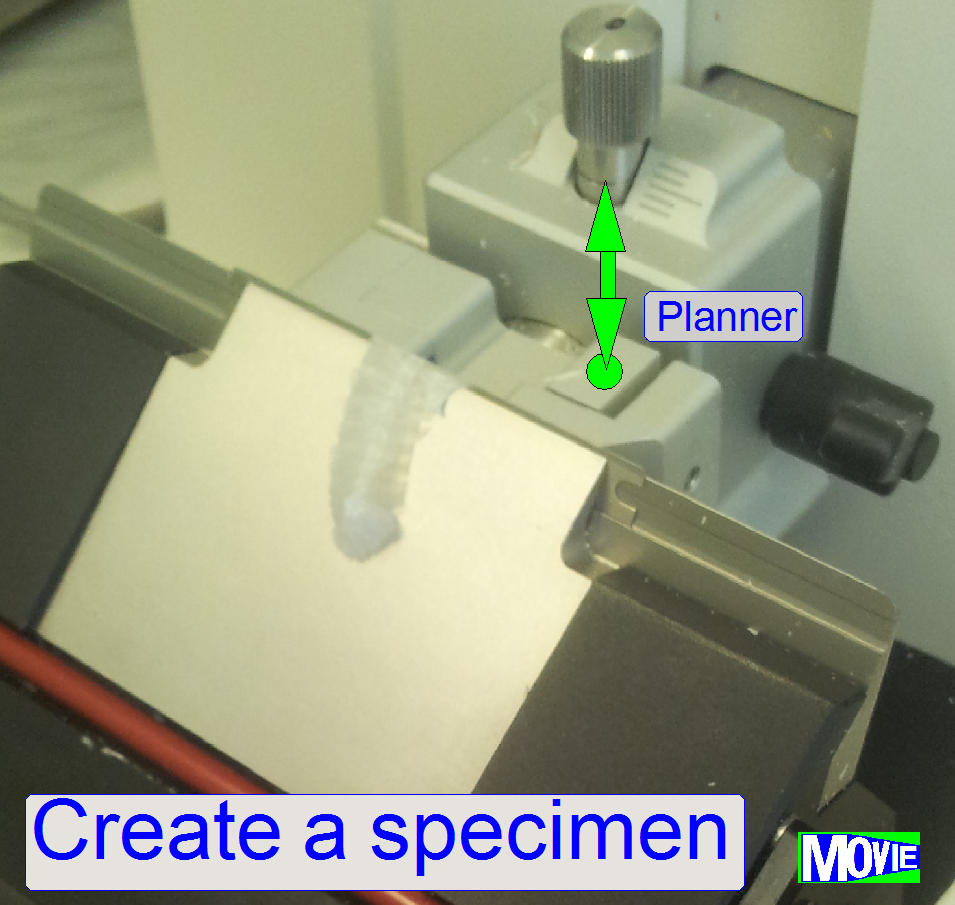
Specimen (sample)
creation process
This process is done to create the specimen and
finally, it will be placed onto the glass slide
·
The creation of the specimen is done before the slide
will be inserted into the slide rack of the iSaSC.
More, detailed information about the sample
creation process can be found in
Introduction
to specimen preparation and
An
animated guide to immunohistochemistry (IHS)
Place slides
The user can fill slides in any bay of the slide
rack; a special sequence is not required.
·
Insert the slide
container
While the process starts, the slide mover senses
3 slide bays for the presence of the slide by using a special algorithm. If a
slide is found, this will be moved to the slide image unit.
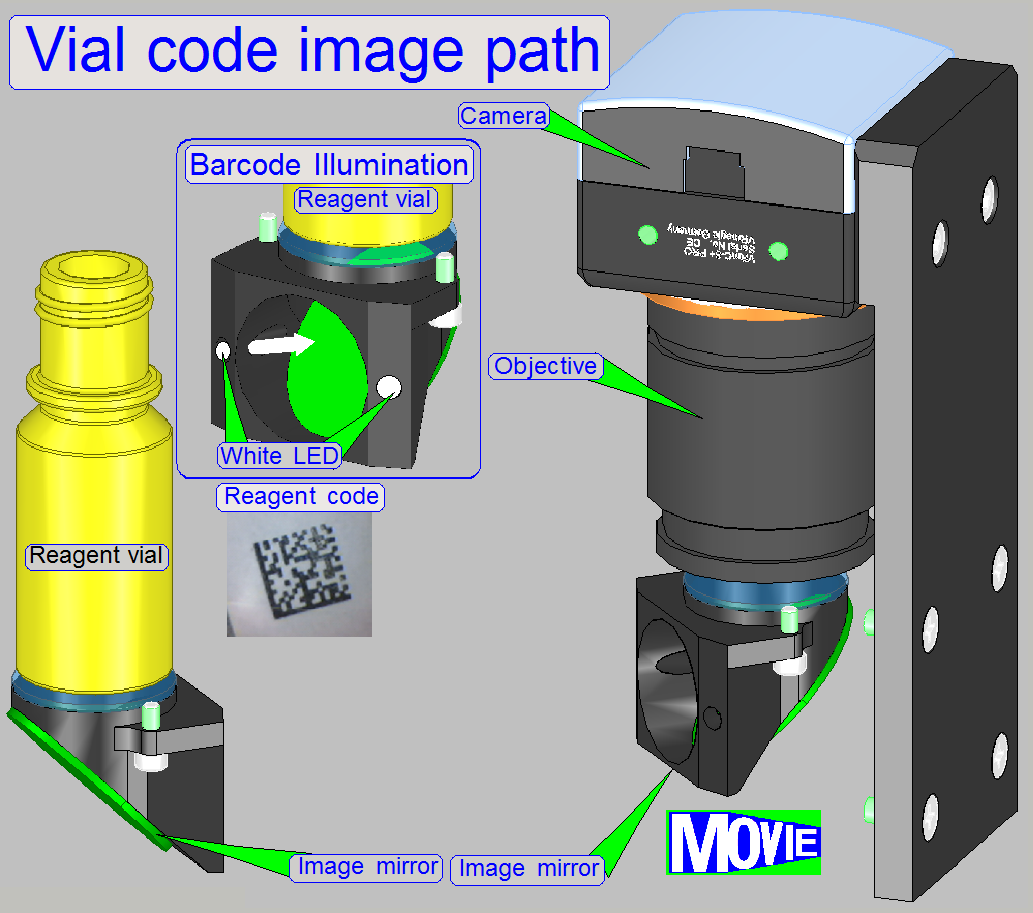
If the image of the specimen (and barcode
capturing of the slide) is made, the slide will be returned to its origin place.
The unit makes an
image of the
·
slide’s scan area and the
·
Barcode area.
This way, the position and the size of the sample
is recognized and the staining procedure will be executed with the entire
tissue. On the other side, because the staining materials are very expensive,
redundant areas of the slide will not be coated with staining fluid.
The barcode of the specimen contains information
about the specimen creation process, dyes, staining and about the scan process
also; how to handle the specimen
The user can define the
· Number of
different dyes for the sample
· Type of each dye
and sequence
· quantity (area) of
the appropriate dye
· residence time of
the dye
After the
residence time of the appropriate dye is passed, the unnecessary dye will be removed
from the sample by washing and rinsing it and drying it up.
If the incubation time is over, the staining
with the next dye follows or, if the staining process is finished, the slide
moves to the cover slipping unit.
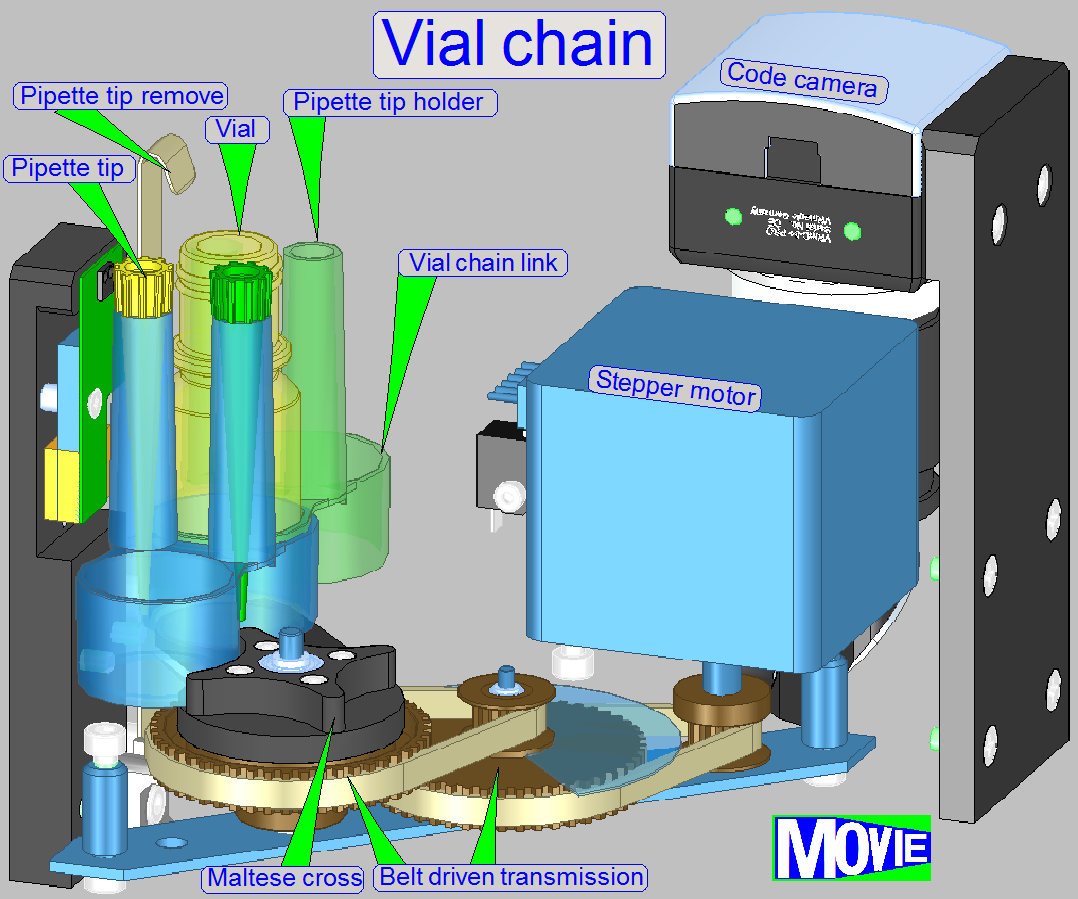
Before the reagent vial is placed into the vial
chain, the reagent code, containing the name, staining time and other process relevant
parameters has to be affixed onto the bottom of the vial.
The software, during the startup procedure reads
the code of each vial and stores process relevant information. Tis way, the
position in the chain is defined also.
Before the staining procedure starts, the
required vial is moved to the vial using position and the pipette tip immerges
into the reagent and gathers the required quantity.
The sample will be stained by dropping dye onto
it.
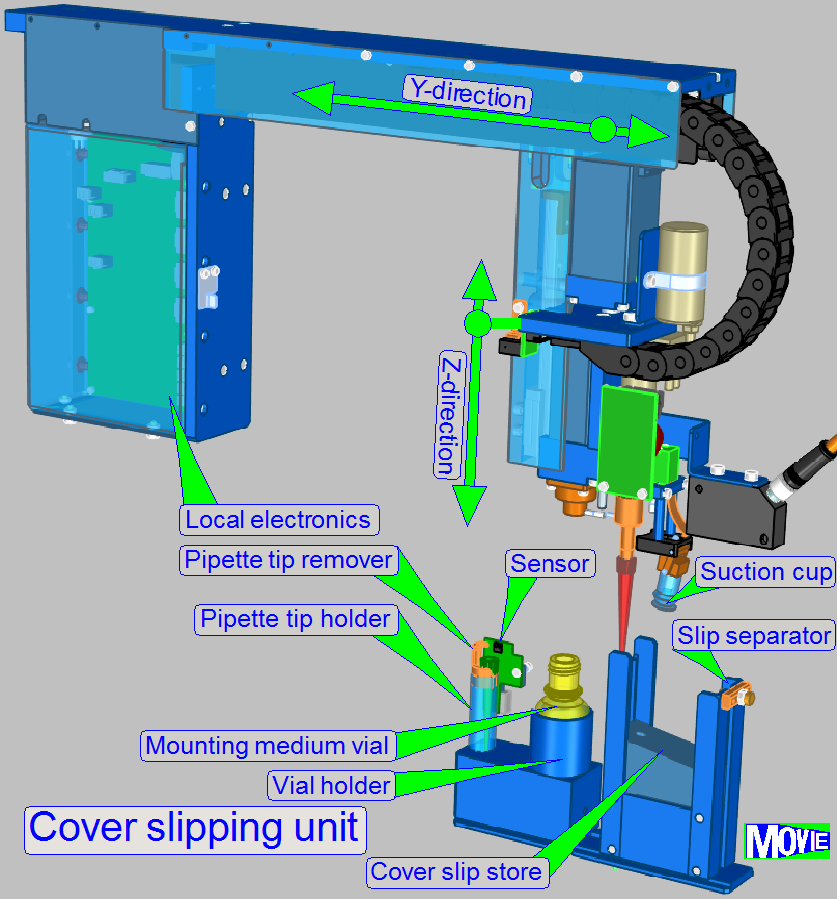
If the rinsing and wiping process of the
specimen is finished, the glass slip will cover the specimen.
With a pipette paraffin will be spend to cover
the specimen and by fitting the cover slip, the paraffin works as an adhesive
bonding mean.
After the specimen
creation process the content of the slide will be scanned.
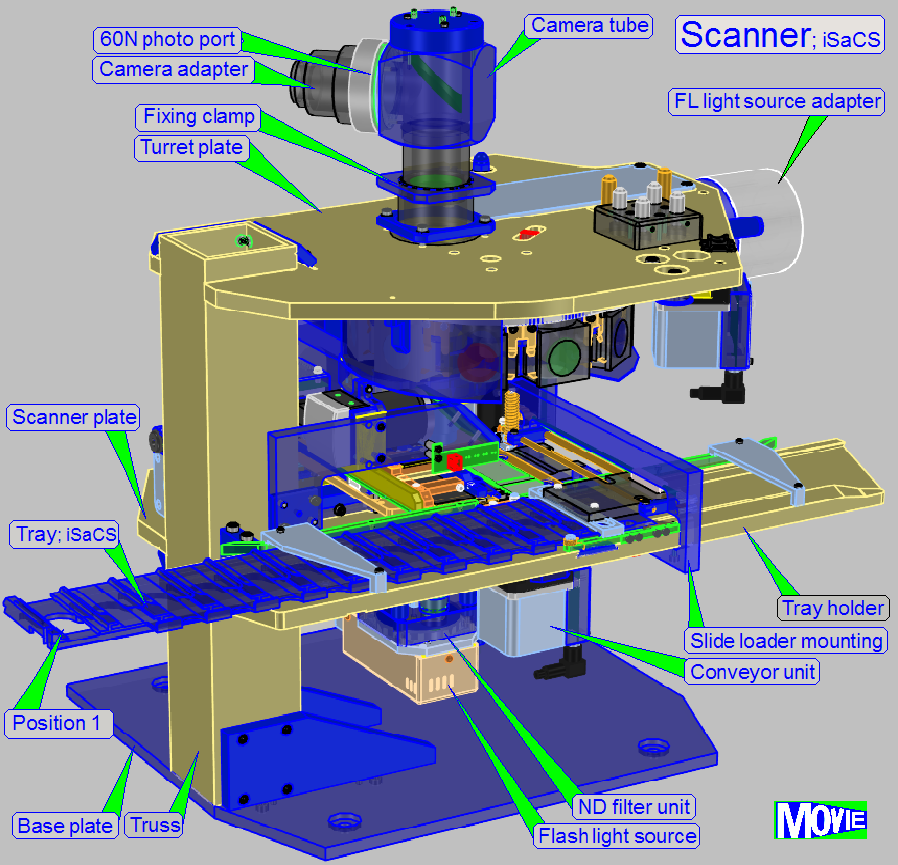
The scanning procedure in the 3DHISTECH iSaCS is
as follows:
· The slides are
inserted into the scanner and removed automatically via a slide holder tray.
· The slide bays in
the slide holder tray allowing an autonomic work of the scanner.
· Designed for
brightfield scan sessions and fluorescent scan sessions.
· The scanner part
is based on a modular
· An automatic scan
session is performed in 30 … 60 seconds for 1 specimen in BF scan mode.