Optics, illumination; M_II
For technicians and
partly for sales managers!
 This chapter handles the components of the brightfield
illumination and the optical path for Pannoramic scanners; previously released
descriptions about the brightfield illumination are no longer valid. Because
our products are developed continuously, some items in the menu may differ to
the actual software version you are using.
This chapter handles the components of the brightfield
illumination and the optical path for Pannoramic scanners; previously released
descriptions about the brightfield illumination are no longer valid. Because
our products are developed continuously, some items in the menu may differ to
the actual software version you are using.
To help resolve problems with the
illumination and optics, a hardware description of the implemented components
and adjustment procedures are added.
Contents
Check the
optical path adjustments
·
For safety regulations regarding
human health and scanner functionality please refer to: Precautions
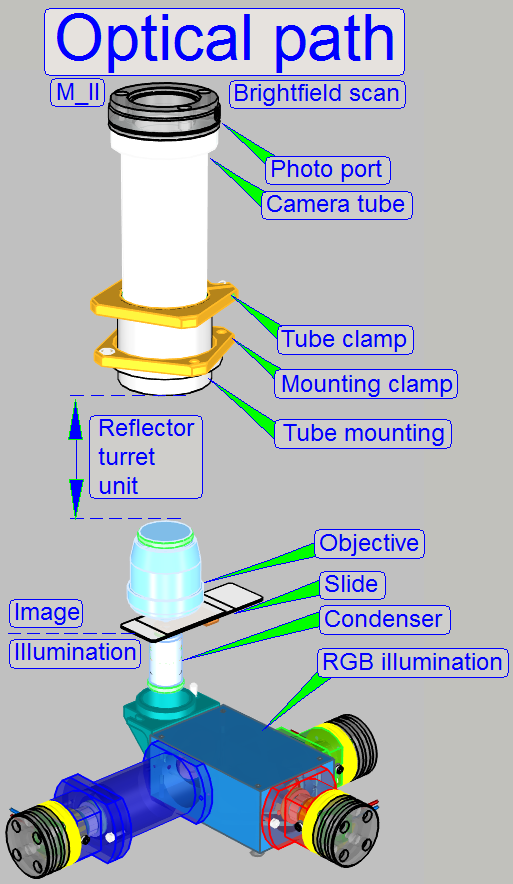 The
BF optical path can be devided principially into an illumination part and an
image part.
The
BF optical path can be devided principially into an illumination part and an
image part.
The border between both is the specimen.
The bottom of the specimen is illuminated, while the
top of the specimen emits lightrays for the image.
Illumination
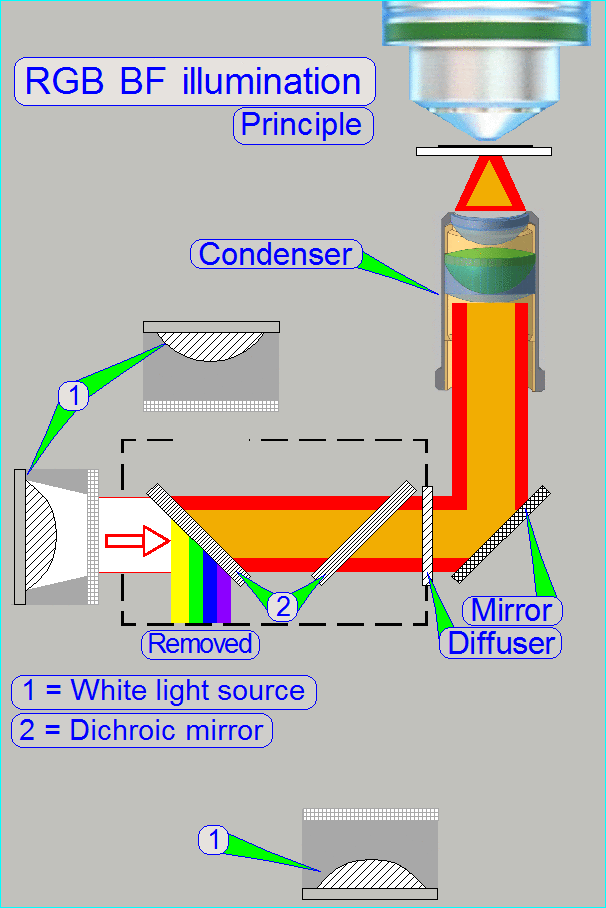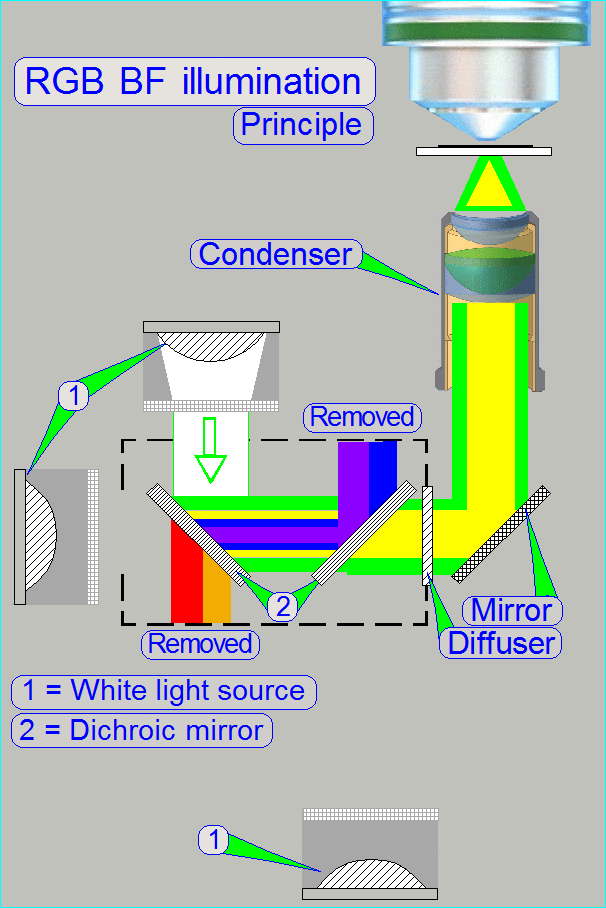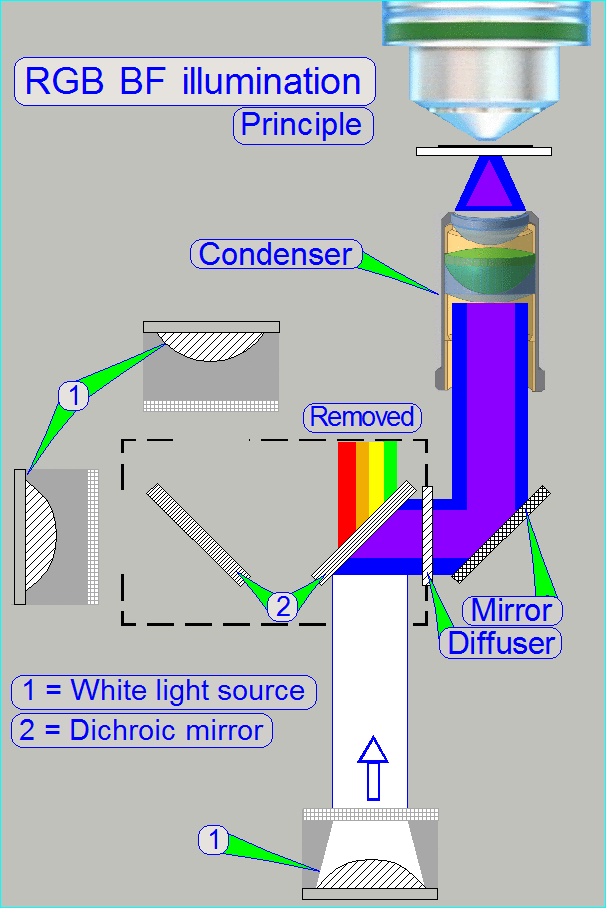
In the MIDI_II the specimen's illumination is done by
an RGB illumination unit, an illumination mirror and the condenser.
The RGB illumination unit creates monochrome light in
the wavelength of Red, Green and Blue, sequentially, so, the specimen's Field
of View is three times illuminated.
The control of the illumination is mainly done by the
shutter time of the camera (triggering), the timing may be done hardware or
software controlled.
The created monochrome, parallel wavelengths are send to the condenser and
this focuses the lightrays to the field of view, observed by the Objective.
Image
The Quality of the image is mainly influenced by its size, resolution,
brightness and contrast.
Size and resolution is influenced by the camera adapter and the sensor
parameters of the scan camera, while other image parameters are mainly
influenced by the construction of the image path, the image illumination and
image magnification.
The objective gathers the light rays, passed through the specimen and
arranges these, together with the tube lens to an image.
The size and resolution of the image may be varied in limits by the
magnification of the camera adapter.
Watch video: Optical path
![]() “Optical path and Field Of View”
“Optical path and Field Of View”
![]() “Influence of the
camera adapter” and “Useable resolutions of scan (main)
cameras”
“Influence of the
camera adapter” and “Useable resolutions of scan (main)
cameras”
The used components are nearly identical in all the three scanner types
(S_M_D_II); but the mechanical construction requires some detailed
modifications. Differences are named as they occur in the description.
 The construction
of the BF optical path uses only a monochrome camera, so only monochrome images
can be produced.
The construction
of the BF optical path uses only a monochrome camera, so only monochrome images
can be produced.
To create color information of the tissue with a monochrome camera, we illuminate
the tissue with monochrome light.
If the tissue is illuminated by blue light, and we are making an image
of the Field of view, the gray scaled camera image contains the intensity of
the blue parts in the tissue.
Because the pixel resolution of the camera is very high and the
resolution of the image's gray scale is 12bit per pixel, very detailed
information of the blue part in the FOV related to the appropriate pixel can be
reached.
If we repeating the procedure with the colors Green and Red, 3 images of
the same FOV are produced and so, the software knows detailed color information
about each pixel of the Field Of View.
By using the software coloring method the true color information of each
pixel is found.
By using cameras with a large image sensor low shutter time and high
pixel resolution (small pixel size), the scan time of the tissue can be held in
acceptable boundaries and the result is an image with high resolution and high
color fidelity.
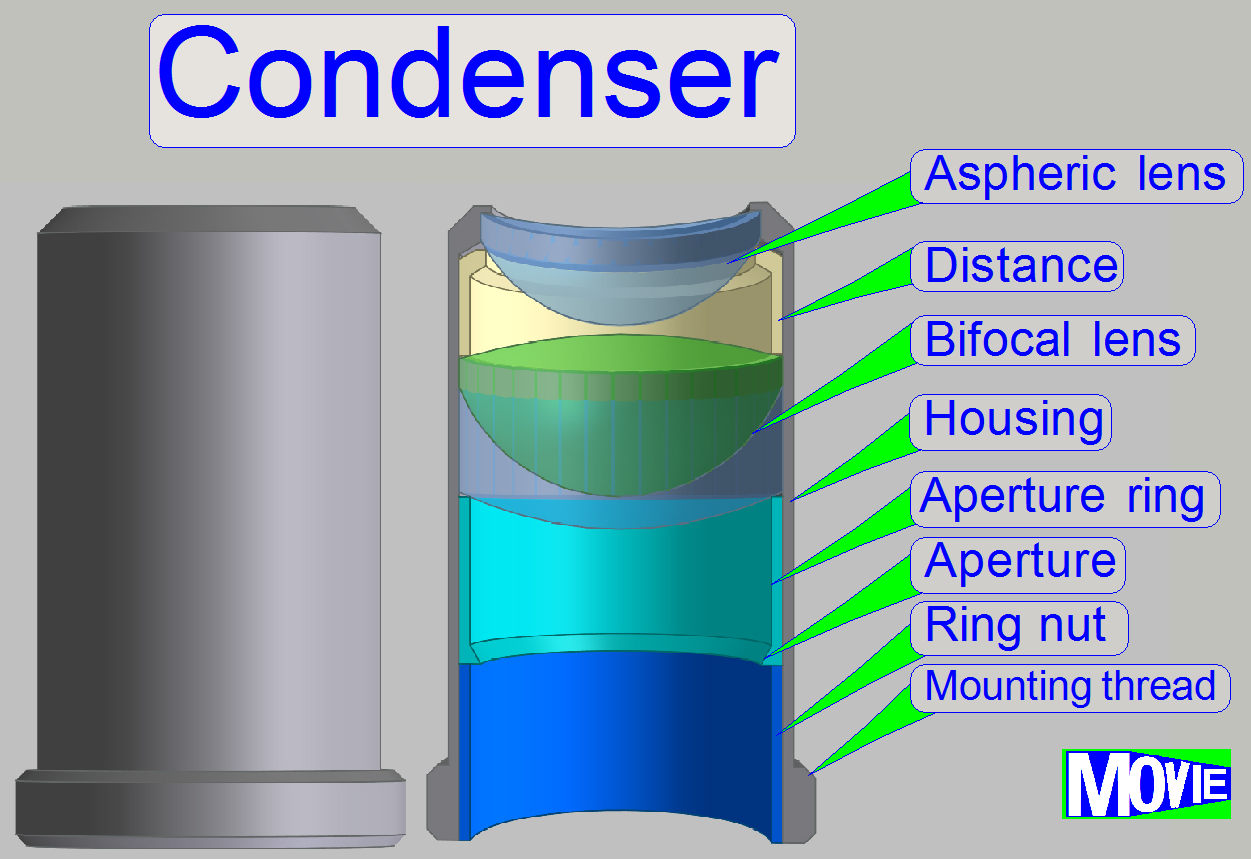 The condenser concentrates the
incoming light to the field of view (FOV).
The condenser concentrates the
incoming light to the field of view (FOV).
Because the size of the illuminated part of the tissue is critical, the condenser
position can be adjusted; the focus position is 10.9mm nominal.
Watch video: Condenser
Remark
The best illumination results would be reached if we would use an
objective also to illuminate the field of view; but because objectives are very
expensive, a condenser is used.
· In optical aspects
we can say, the condenser is a simplified objective.
·
See also “Focus unit”.
·
See also “Adjustment
procedures”.
![]() Condenser ; Wikipedia
Condenser ; Wikipedia
![]() Condenser; ©
microscopy-uk.org
Condenser; ©
microscopy-uk.org
 In microscopes, the objective gathers the light, emitted
from the tissue to be observed and focuses the rays to produce an image. The
character of the objective is given by the magnification and the
numerical aperture.
In microscopes, the objective gathers the light, emitted
from the tissue to be observed and focuses the rays to produce an image. The
character of the objective is given by the magnification and the
numerical aperture.
The position of the objective and
the distance to the tissue is very important to produce a sharp image. Because
in Pannoramic scanners this distance can be modified by moving the tissue position
(focusing) both positions are important, the objective position
and the nominal focus
position.
Remark
In the standard version of SCAN,
·
See also “the focus
unit”.
·
See also “Adjustment
procedures”.
·
To exchange the
objective, please refer to the appropriate chapter “Exchange the objective” in
the chapter “How
to exchange spare parts and units”
![]() “Optical path and Field Of View”
“Optical path and Field Of View”
![]() Objective; © Objectives_for_Microscopes_from_Carl_Zeiss.pdf;
stored
Objective; © Objectives_for_Microscopes_from_Carl_Zeiss.pdf;
stored
Slide, tissue and cover slip
Important
If the scan program takes the compensation images after the BF part of
SlideScanner.exe was started and the program stops with the error message
· “The parameter is incorrect”,
please check the
components of the optical path; the camera exposure time is outside the allowed
range!
· Condenser inserted and condenser
position is correct
· No filter block inserted in the
optical path (10th filter wheel position) and the filter wheel
hardware limits are set correctly
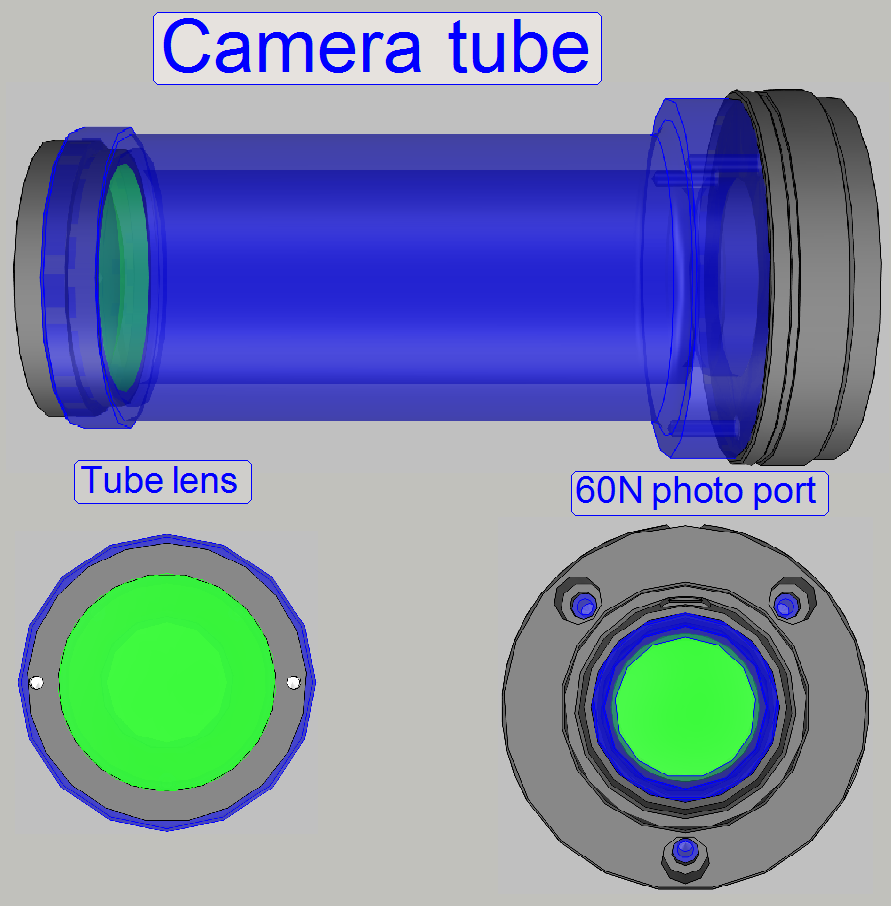
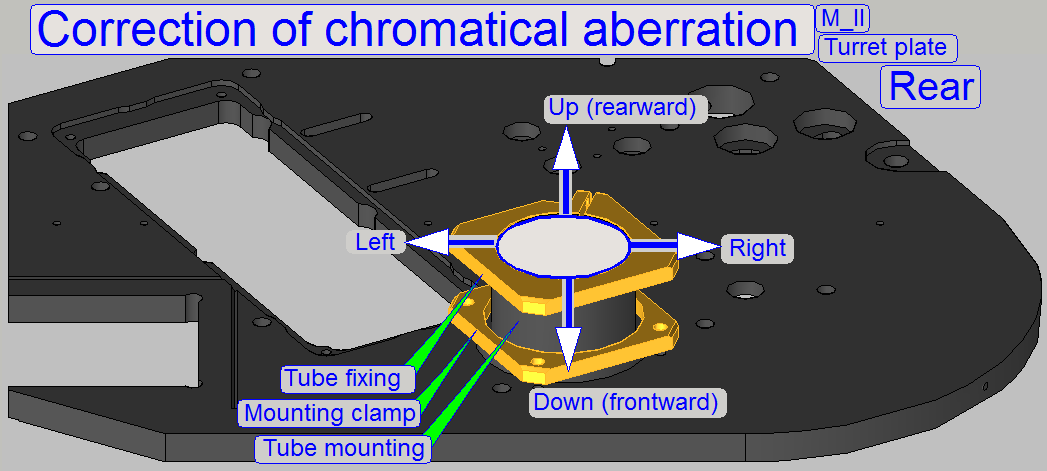
Camera tube mounting; SCAN,
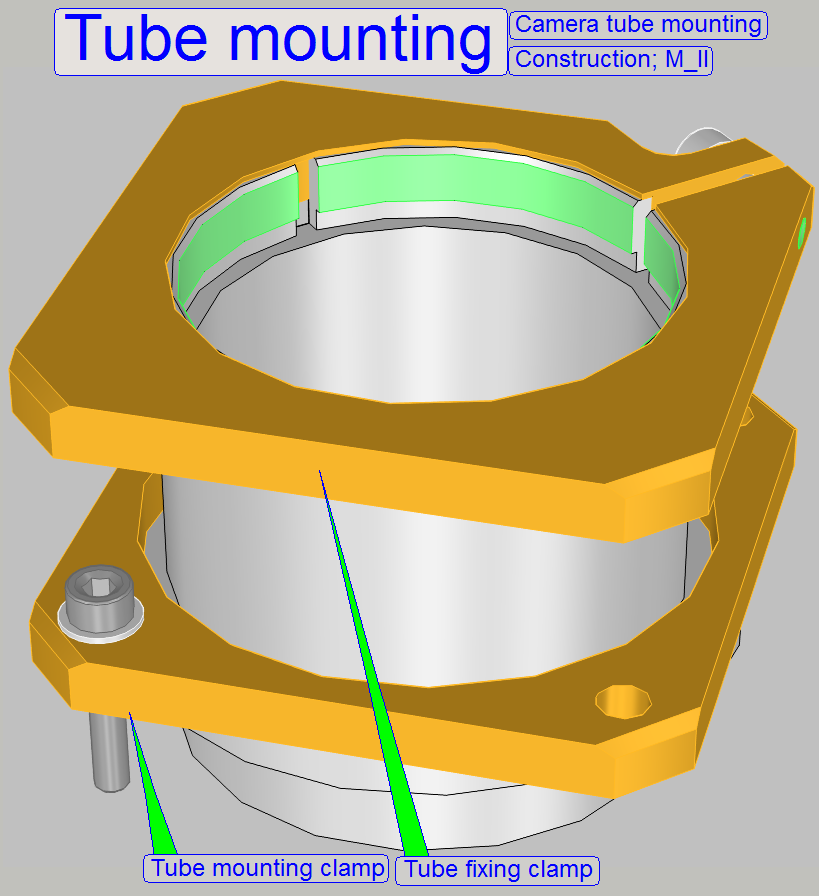 The tube is mounted to the turret unit (or the turret
plate) so, that the correct position can be adjusted. By moving the tube
mounting into the center of the optical path, the chromatic
aberration is corrected and minimized.
The tube is mounted to the turret unit (or the turret
plate) so, that the correct position can be adjusted. By moving the tube
mounting into the center of the optical path, the chromatic
aberration is corrected and minimized.
·
For
adjustments, loosen the four clamp mounting bolts to make the tube mounting
barely moveable.
![]() “Adjustment procedures”.
“Adjustment procedures”.
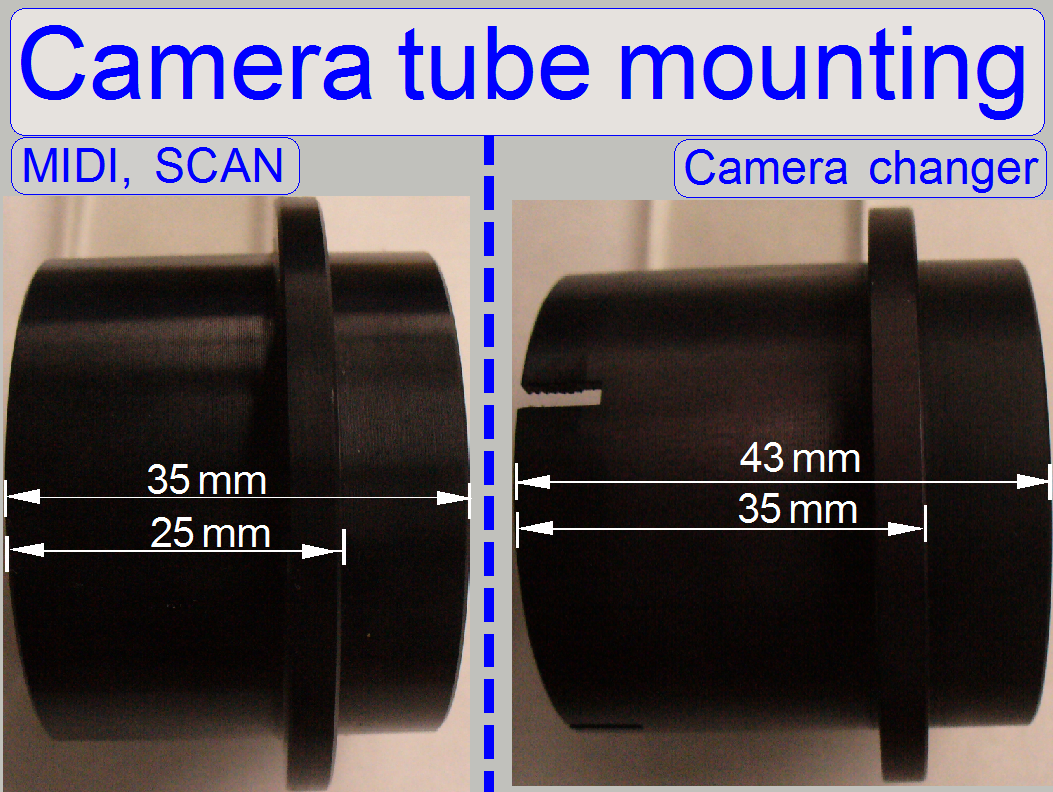
- The tube mounting for SCAN and MIDI-type scanners
is different in size in relation to the tube mounting of the camera tube
for the camera changer unit!
- Check the correct size if the turret unit was
exchanged!
- Never mix the tube mounting in the systems!
On the side, near to the objective, the tube lens is situated; this
performs the image (together with the objective). Into the space between
objective and tube lens further optical components can be inserted, like the
filter block for the fluorescent scan or a image mirror like in the DESK. For best image quality, the tube lens should
be mounted into the camera tube until it stops!
The camera adapter 60 C1” can be also connected to the 60N photo port.
![]() “Optical path and Field Of View”
“Optical path and Field Of View”
The camera adapter is situated
between the camera tube and the scan camera and offers the possibility to
insert lenses or other optical means like filters into the image path.
If lenses are inserted, the camera adapter modifies the image size, the magnification
and the resolution of the image.
The usable magnification of the camera adapter depends on the scan
camera’s CCD size, its resolution and the construction of the optical path.
Watch video: Camera adapters
![]() Camera adapter CARL
ZEISS; micro shop
Camera adapter CARL
ZEISS; micro shop
Influence of the camera adapter
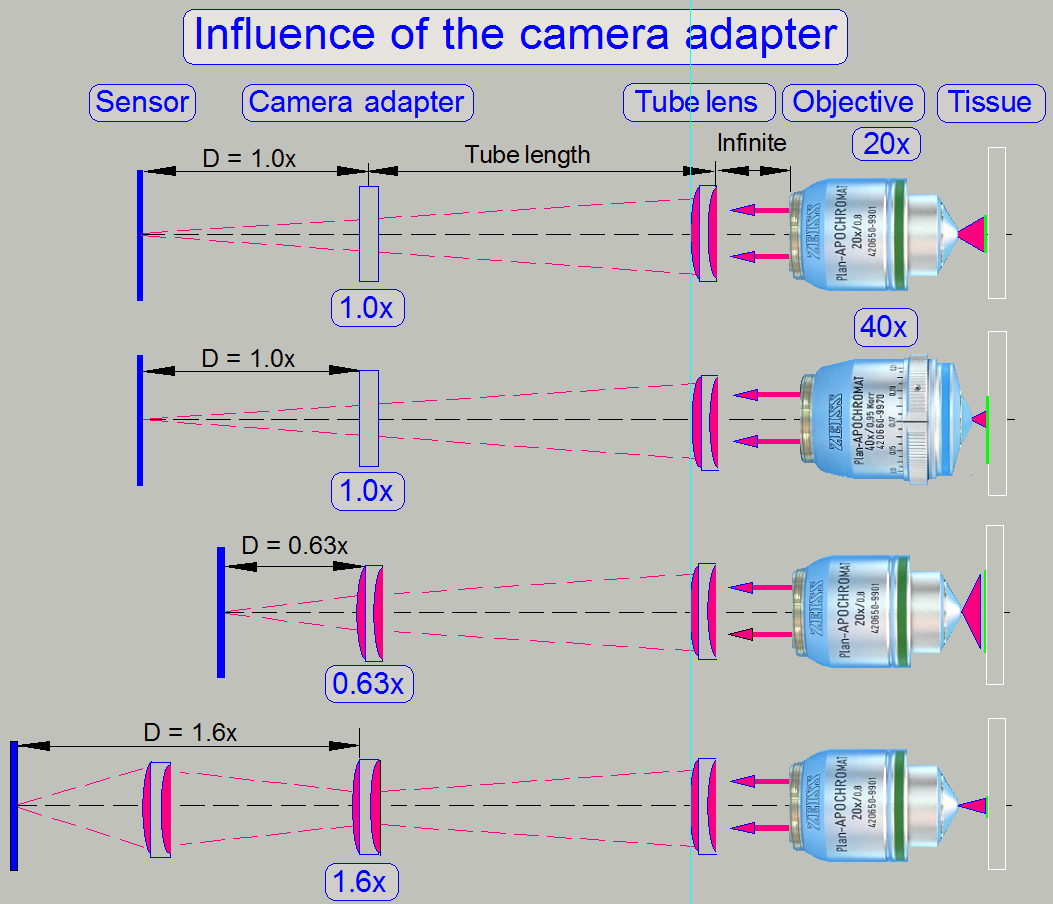
The useable magnification of the camera adapter depends on the size of
the camera's sensor (useable geometry x and y in pixels), the used objective
magnification and the construction of the image path (Length of the camera
tube).
· The resulting
magnification of the image path is defined by the product of Objective
Magnification multiplied by the Camera Adapter Magnification.
Example
If the Objective Magnification is 20x and the camera adapter
magnification is 0.63x the resulting magnification of the image path will be
12.6x.
Image
magnification = 20 x 0.63 = 12.6
Advantage
By reducing the image
magnification, the dimension of the FOV will be increased; the scan speed
increases because the number of FOVs to be scanned is reduced.
Disadvantage
The resolution of the virtual
tissue is reduced.
Conclusion
· The camera adapter
fits the image, seen by the objective in the focus of the camera sensor (with
its length) and influences the resulting magnification of the image path and
the size of the FOV.
· If the camera
adapter magnification is 1x, then no lenses are inserted, and the sensor is in
the focus of the tube lens; the optical magnification is defined by the
objective magnification.
· If the camera
adapter magnification is 0,63x, then the lens of the camera adapter enlarges
the FOV; the resolution of the scanned tissue is decreased.
· If the camera
adapter magnification is 1,6x, then the optics of the camera adapter makes the
FOV smaller, and the resolution of the scanned tissue is increased!

The charge
coupled device (CCD) of the scan camera transforms the incoming light (the
image) into electrical charge; and this is read out by the electronics of the
camera.
In the MIDI_II the default camera is the monochrome "Grasshopper
3", manufactured by POINTGREY.
·
![]() “Prerequisites”,
usable scan
cameras, and Grasshopper3.
“Prerequisites”,
usable scan
cameras, and Grasshopper3.
·
![]() “Adjustment procedures” to “Adjust the
camera rotation angle”.
“Adjustment procedures” to “Adjust the
camera rotation angle”.
Optical path and Field Of View
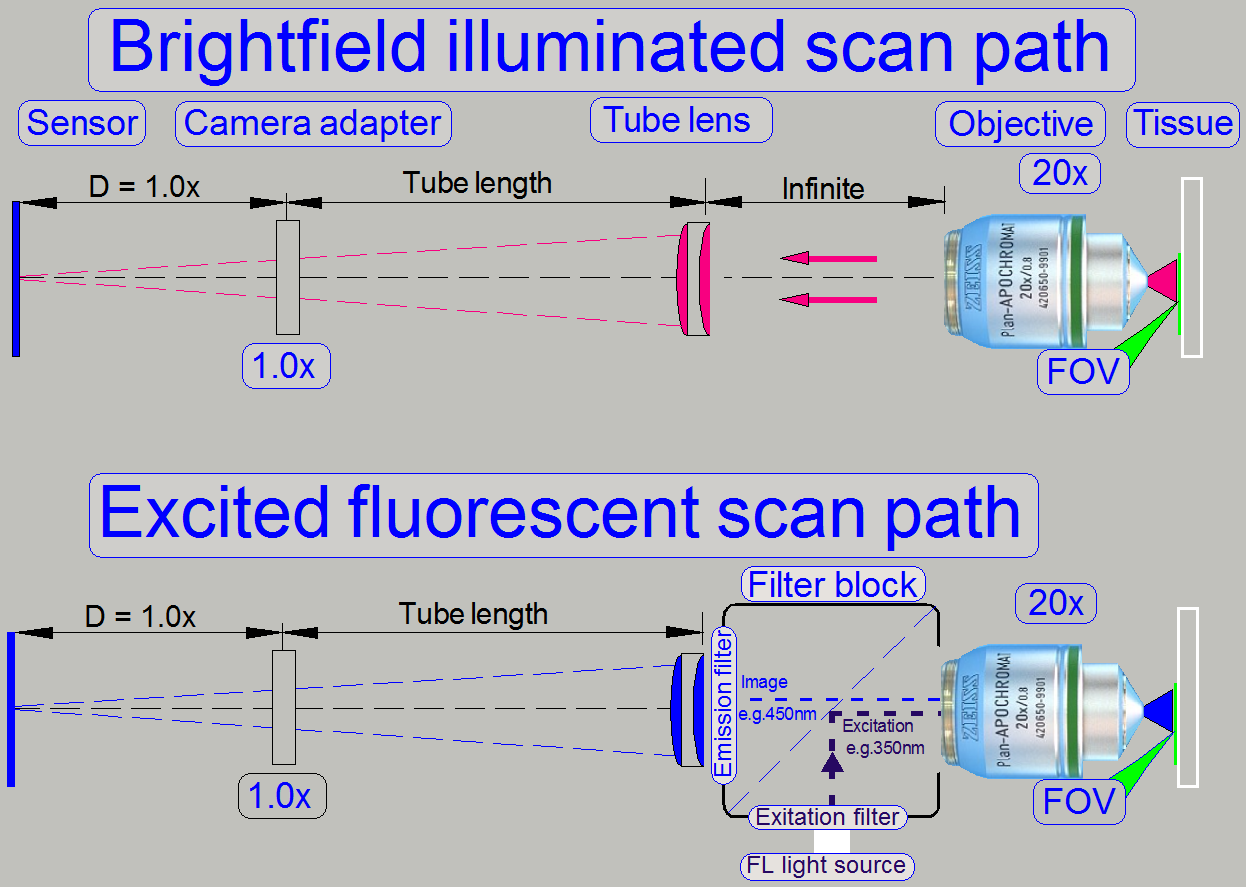
The pupil of the scan objective is very close to the tissue, so, the
small area on the tissue will be enlarged by the objective and the camera
adapter.
The seen area on the slide is always defined by the size of the camera’s
sensor; more precise, the effective number of pixels horizontal and vertical
and the optical means in the image path.
The objective type “Plan-Apochromat” requires a tube lens to create the
image. In opposite to other objective types, an infinite space exists between
the objective and the tube lens, in which
the light rays are parallel.
So, optical means, like the image mirror in the DESK type scanners or
the filter block
in fluorescent
scan sessions can be inserted (by the help of the turret unit)
· The filter block’s
components do not affect the magnification of the image path!
General
Even illumination is important in
microscopes and in all of our scanners as well. A well adjusted illumination
ensures that any approved camera can be used properly with our scanners without
further adjustments.
The entire adjustment procedure can
be divided into two main parts,
1. The FOV illumination adjustment and
2. The image path adjustment.
The adjustment parts can be done
nearly separately from each other, but always the illumination path is adjusted
first and only then will be adjusted the image path. If the adjustments are
done, the entire result should be checked again!
The adjustment is always done from
the light source to the tissue and from the tissue to the CCD of the camera.
Because distances are not measureable, the actual adjustment result is used to
adjust the next component. This procedure requires adjusting or checking the
position of previously adjusted components again!
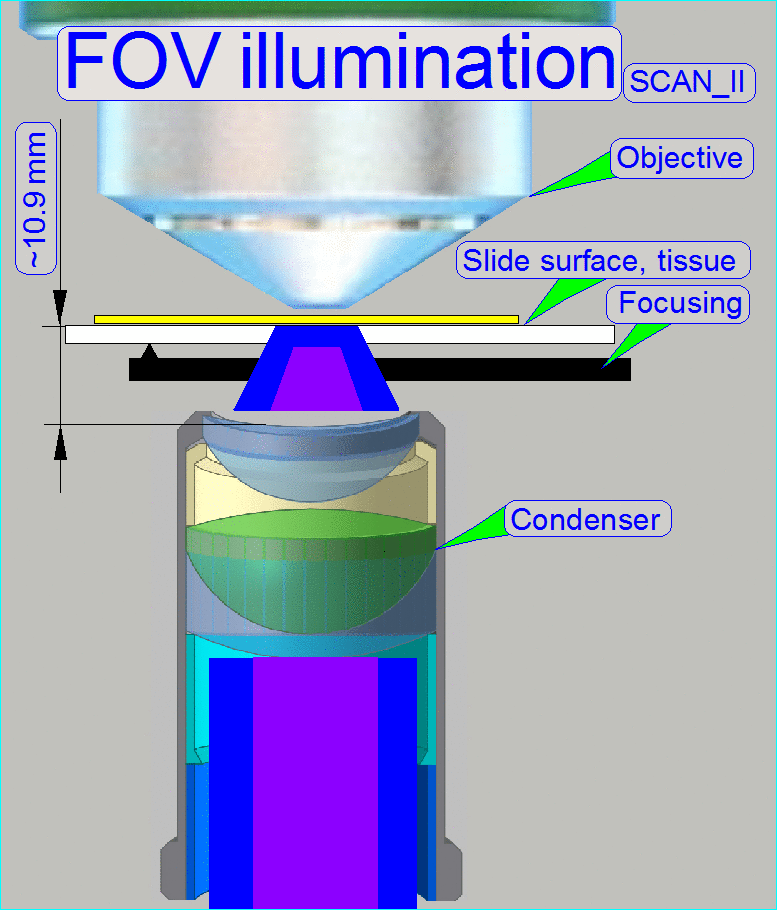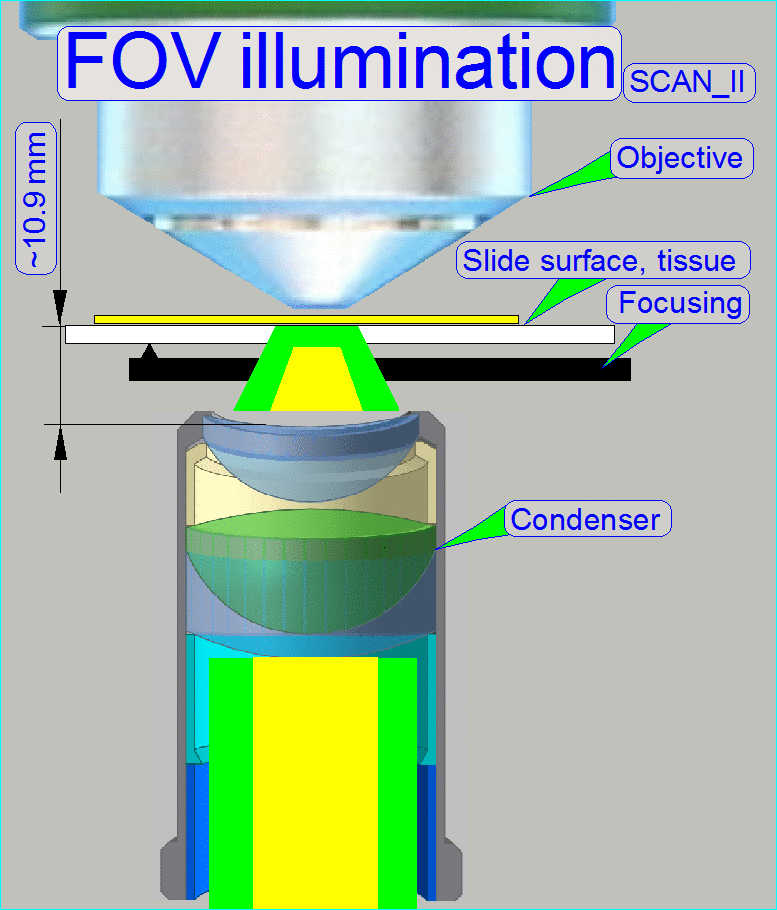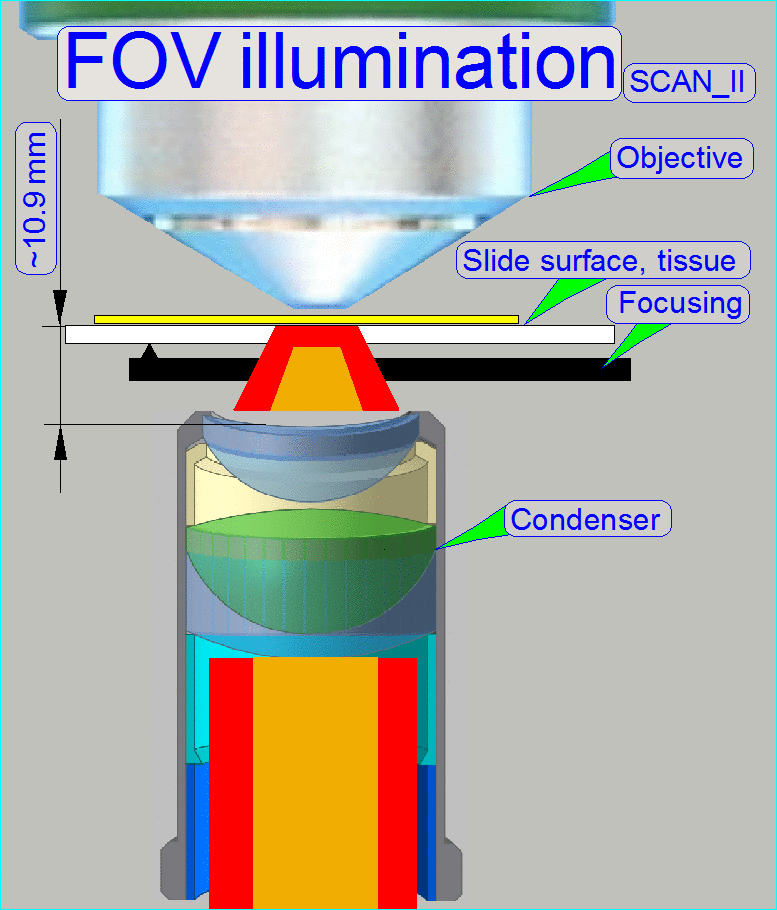
The goal of the brightfield
illumination adjustment is, to illuminate the FOV, seen by the objective
pupil (and the scan camera) evenly and with a density of light as much as
possible.
·
If the FOV is
not fully and evenly illuminated, the quality of the virtual tissue becomes
poor (“Stripping”
or “Color shading” occurs), and
·
If the
illuminated field is too large, the exposure time of the camera will increase
and the scan procedure slows down, because the light density is reduced.
Because the image, delivered by the
scan camera is used for the adjustments of the illumination path, some
adjustments (Objective- and focus position) for the image path have to be done
before the illumination can be adjusted.
Furthermore, because we using colors
to adjust the illumination path, the final correctness of the illumination path
must be checked with the LUT-adjustment again, after the chromatic
aberration and the
camera rotation angle is adjusted.
![]() Brightfield
Microscopy, Wikipedia
Brightfield
Microscopy, Wikipedia
Adjust the objective and focus position
 This Adjustment is no longer required in S_M_II-type
scanners.
This Adjustment is no longer required in S_M_II-type
scanners.

![]() “Mount the objective” and “Focus unit”.
“Mount the objective” and “Focus unit”.
 The adjustment of the
condenser is important for the bright, uniform and optimal illumination of the
FOV. This reduces so the exposure time of the camera and increases the quality
of the scanned tissue. If the objective position was modified, the correctness
of the condenser position has to be checked again!
The adjustment of the
condenser is important for the bright, uniform and optimal illumination of the
FOV. This reduces so the exposure time of the camera and increases the quality
of the scanned tissue. If the objective position was modified, the correctness
of the condenser position has to be checked again!
The position of the condenser affects the following:
· The size of the
visible FOV (color shading)
· The image contrast
·
The image
resolution (the numerical aperture) and
· The exposure time.
1. Create a live view
with the scan camera in the focus tab and set the focus motor position to 1600 steps.
2. With the preview positioning tool ![]() find a
“clean” FOV outside the tissue and inside the cover slip, without dust.
find a
“clean” FOV outside the tissue and inside the cover slip, without dust.
3.
Loosen the condenser’s fixing bolt.
4.
Rotate the condenser toward to the
objective, to find the start position for the adjustment; the brightness will
increase.
5.
Rotate the condenser in opposite direction
(away from the objective) and look at the live view. Beware of the condenser
cover (shutter), don’t close it and don’t bend it. You will see two surfaces
(from the diffuser) coming into focus (see “Condenser 1 and
6.
 After the second
surface just disappeared (Condenser 2) and the live image is smooth and bright,
stop moving the condenser and tighten its fixing bolt, see “Condenser position”
(the pictures was done with previously adjusted illumination. If you are
starting the adjustment, the figure “Condenser position” might be is not so
smooth).
After the second
surface just disappeared (Condenser 2) and the live image is smooth and bright,
stop moving the condenser and tighten its fixing bolt, see “Condenser position”
(the pictures was done with previously adjusted illumination. If you are
starting the adjustment, the figure “Condenser position” might be is not so
smooth).
7.
If the brightness decreased too much, repeat the steps
10 to 13.
8.
Check the correct
condenser position in the focus positions 1200, 1600 and 2000 steps. There must
not be significant differences.
The entire image path adjustment includes
the adjustment of the following parts:
1. The objective position
This
adjustment is in M_II scanners no longer required.
2. Camera tube position
The
position of the camera tube (lens) affects the color trueness of the scanned
tissue; the chromatic aberration becomes visible in more blue, and more red or
yellow colored cell borders on the opposite sides; see also “Chromatic
aberration” and “Adjustments”.
3. Camera rotation angle
If
the camera rotation angle is out of the limits, the stitching is not correct
and the borders of the FOV’s becoming visible in the virtual tissue with the
viewer program, the sample does not fit on the border of the FOV; see also
“Stitching’.
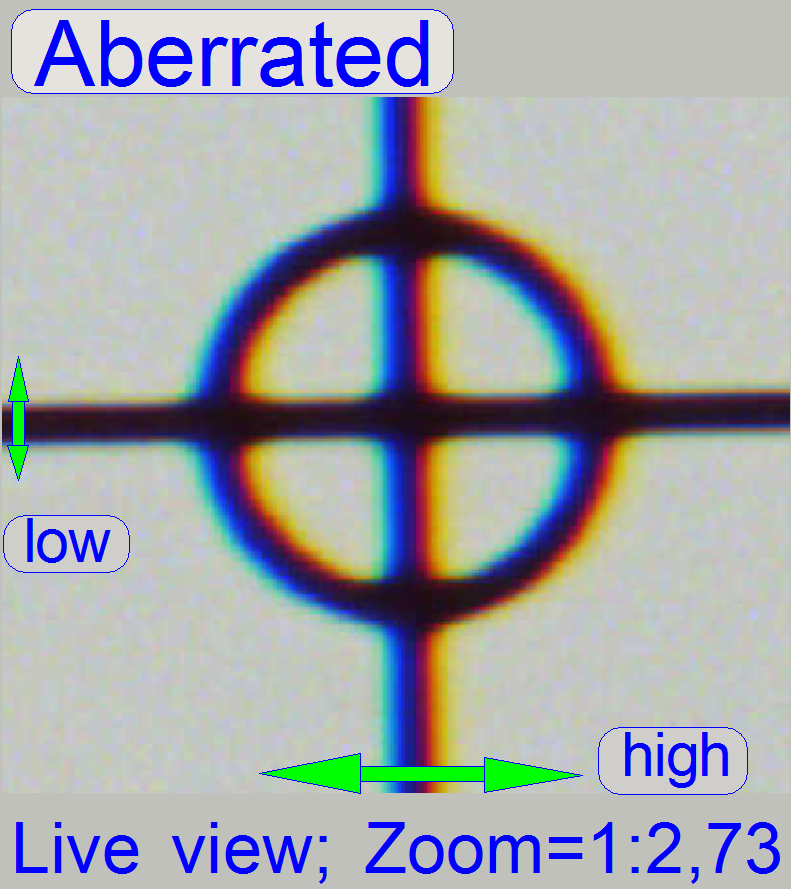 The appearance of
chromatic aberration can be divided into two main reasons:
The appearance of
chromatic aberration can be divided into two main reasons:
1.
The used materials (the composition of the glass) in the
lens system; different wavelengths of light will be focused to different
positions; and
2.
The arrangement of the lenses to each other
(centermost), with other words, the straightness of the optical path (lens
system).
· For any kind of
optical aberration see “Optical
aberrations”
Chromatic aberration of a FOV is seen as unevenly colored cell borders.
Because the first item is given by the used optics (the
construction of the objective and lenses) and can not be affected by the
technician, we minimize the chromatic aberration by making the optical path
straight and centered.
For this purpose, in the SCAN and the
- After the
chromatic aberration adjustment was finished, the camera rotation
angle has to be adjusted (again).
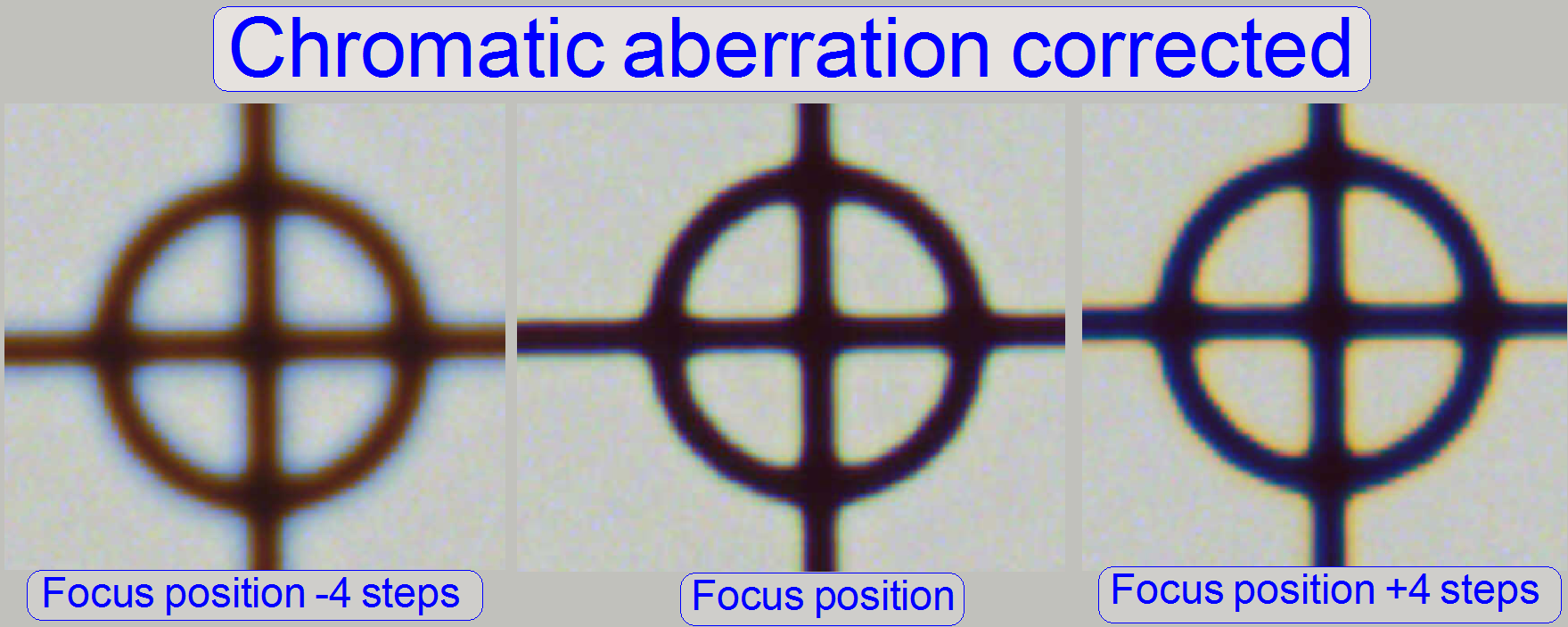 The adjustment of the chromatic aberration is done in
the real focus position and in the center of the FOV to be observed. To check
the result of the adjustment, the focus position can be modified by some steps
in positive or negative direction. In this way, the correctness of the
adjustment becomes more visible. If the yellow color occurs evenly on the inner
and outer part of the circle in the center, the adjustment is acceptable; see
“Focus position +4 steps”.
The adjustment of the chromatic aberration is done in
the real focus position and in the center of the FOV to be observed. To check
the result of the adjustment, the focus position can be modified by some steps
in positive or negative direction. In this way, the correctness of the
adjustment becomes more visible. If the yellow color occurs evenly on the inner
and outer part of the circle in the center, the adjustment is acceptable; see
“Focus position +4 steps”.
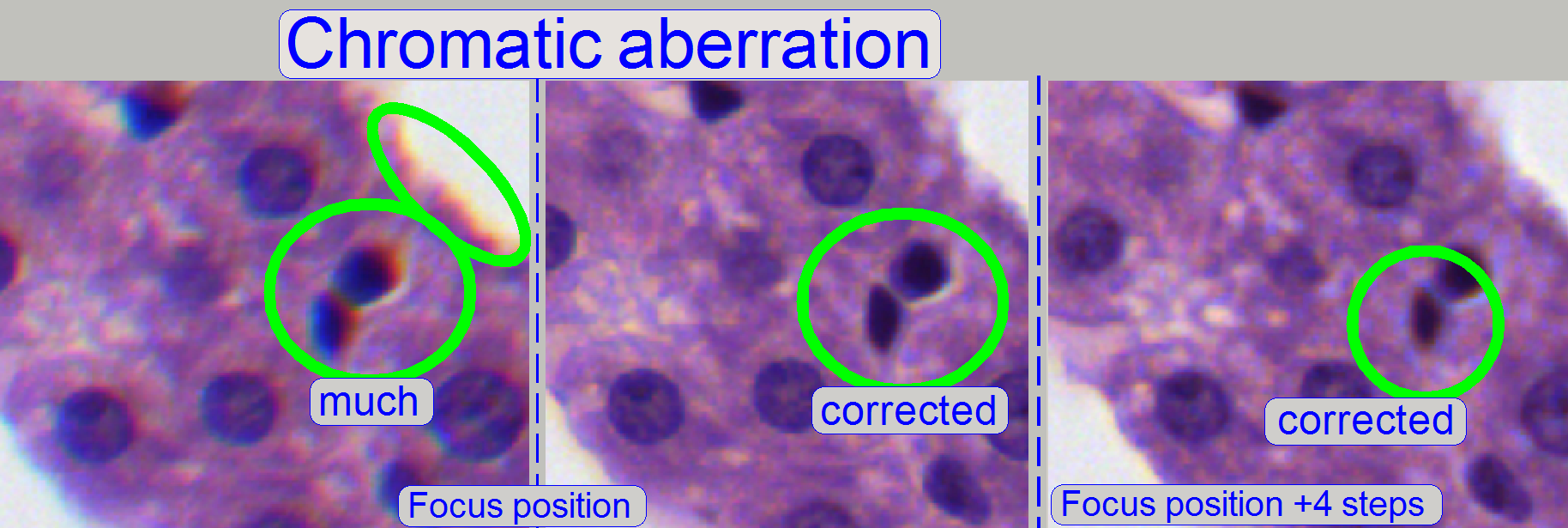 The images was done in the focus position of the live
view, except otherwise specified and with a zoom factor of 2,73
The images was done in the focus position of the live
view, except otherwise specified and with a zoom factor of 2,73
Reduce the chromatic aberration
 Chromatic aberration becomes visible if the optical
light path is not exactly perpendicular (mirrors) or not fully centered
(lenses); it is corrected by different positioning of the tube (mounting). For
this purposes use a well visible tissue. This adjustment assumes that other optical adjustments are already
finished! To adjust the chromatic aberration use and observe always the center
of the FOV, never the border, because the border has always more chromatic
aberration as the center!
Chromatic aberration becomes visible if the optical
light path is not exactly perpendicular (mirrors) or not fully centered
(lenses); it is corrected by different positioning of the tube (mounting). For
this purposes use a well visible tissue. This adjustment assumes that other optical adjustments are already
finished! To adjust the chromatic aberration use and observe always the center
of the FOV, never the border, because the border has always more chromatic
aberration as the center!
Example: If the otherwise dark spots in the tissue have
blue boundaries on the top, and red or yellow on the bottom (see also above “Chromatic aberration”),
move the tube to the red (yellow) direction.
![]() Reduce the chromatic aberration
Reduce the chromatic aberration
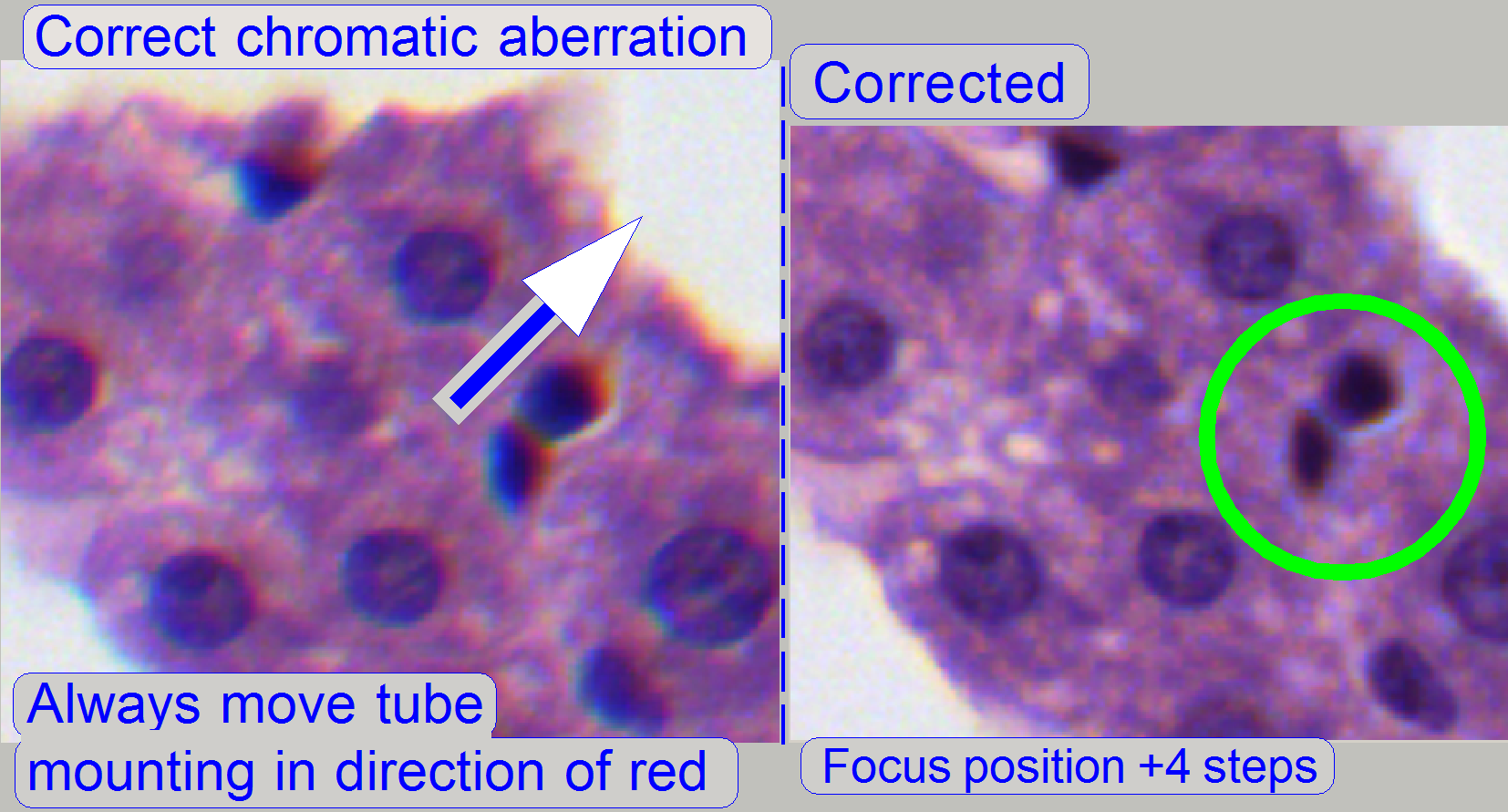 By barely loosening
the bolts of the mounting clamp, the tube mounting can be moved to reducing the
chromatic aberration. Move the tube mounting always in the direction of red
until chromatic aberration is no longer visible.
By barely loosening
the bolts of the mounting clamp, the tube mounting can be moved to reducing the
chromatic aberration. Move the tube mounting always in the direction of red
until chromatic aberration is no longer visible.
- Do the correction with zoom=2,73 and in the
center of the FOV.
- Use the option "Cross line on
image" in the live view and move the observed cell(s) into the center
of the image.
- By moving the focus motor some steps away
from the focus position, the correctness of the adjustment becomes more
visible.

1.
Start the program “SlideScanner.exe” and load a slide
with tissue.
· Important: Check the proper position of the slide in the specimen
holder.
2.
After the preview is done, select the option Focus and
click on the button “Live view”, positioning tool ![]() and click inside the tissue and find a well
usable FOV with a lot of cells. Use the “Auto focus” button.
and click inside the tissue and find a well
usable FOV with a lot of cells. Use the “Auto focus” button.
3.
 Fit the camera view
to window size with the button 1:1 and zoom in by using the zoom tool until a
zoom value of 2,73 is reached. By moving the horizontal and the vertical scroll
bar to the middle of their acting range, the center of the FOV is in the center
of the screen.
Fit the camera view
to window size with the button 1:1 and zoom in by using the zoom tool until a
zoom value of 2,73 is reached. By moving the horizontal and the vertical scroll
bar to the middle of their acting range, the center of the FOV is in the center
of the screen.
4.
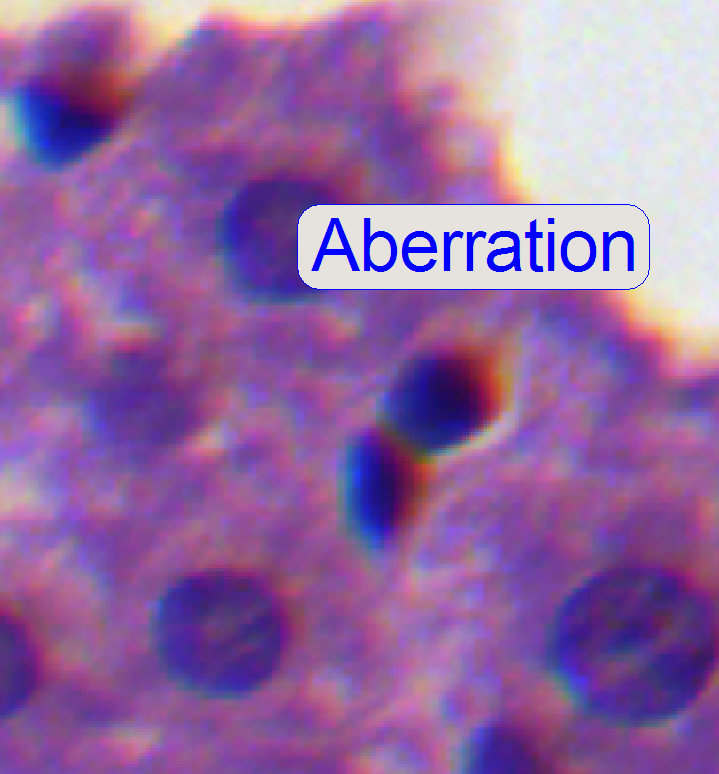 If the zoom value
is large enough (between 2.6 and 3), you can see something like this
“Aberration”. If yellow, red or brown colors are visible at the boundaries of
spots, the optical system has chromatic aberration; check this behavior on different
positions of the tissue also.
If the zoom value
is large enough (between 2.6 and 3), you can see something like this
“Aberration”. If yellow, red or brown colors are visible at the boundaries of
spots, the optical system has chromatic aberration; check this behavior on different
positions of the tissue also.
Procedure for SCAN and
5.
Loosen the tube fixing
bolts until the tube becomes just barely moveable.
6.
Move the tube in the direction, where the red or
yellow color of the spot or cell occurs. (With the Pannoramic SCAN: Take into
account, that the camera is mounted parallel to the magazine loader edge (30
degrees), so that the directions up, down, left and right are also turned 30
degrees; see also “Camera
rotation angle”. Remember, the chromatic aberration will be
adjusted always in the center of the field of view!
7.
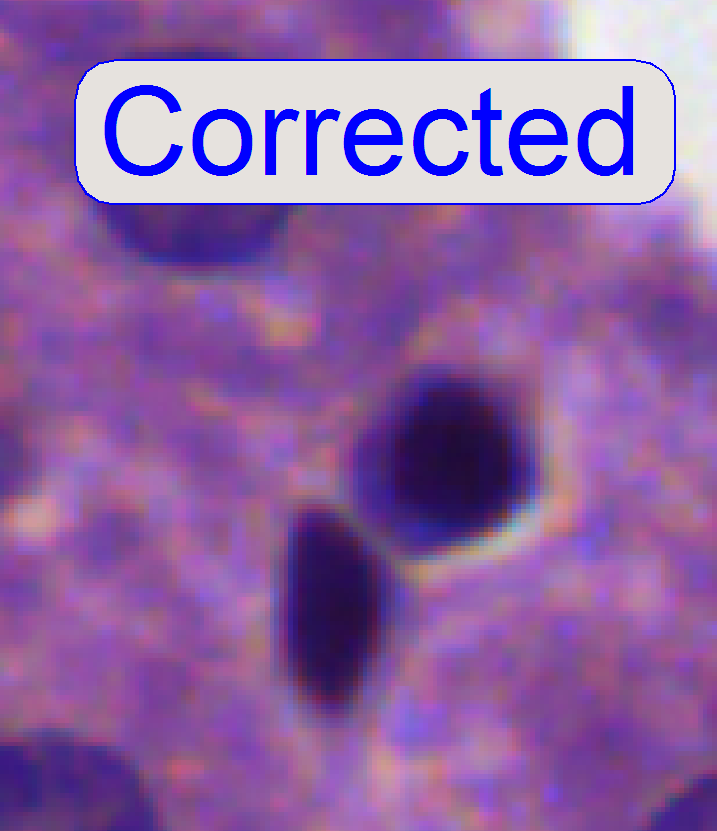 After pressing the
button “auto focus”, use a focus step size of 2 steps and go from the auto
focus position in plus direction. If the cell gets a brown or yellow ring in
nearly constant thickness the aberration seems to be adjusted.
After pressing the
button “auto focus”, use a focus step size of 2 steps and go from the auto
focus position in plus direction. If the cell gets a brown or yellow ring in
nearly constant thickness the aberration seems to be adjusted.
8.
Repeat step 7 and check this result on different
positions of the same slide (tissue) with live view.
9.
Scan a tissue or a part of it and check the result
with the SlideViewer. When you can find more positions where the aberration is
visible always on the same side of the cells, repeat from step 6 (if DESK then
from step “b”).
10.
When you can find parts of the tissue where the
chromatic aberration is visible on different sides of the spots, the chromatic
aberration seems to be adjusted.
11.
Scan two further tissues with different samples and
check the results (repeat the steps 9, 10).
12.
If the boundaries of the spots (see “corrected”) are
colored evenly the optical path is correct.
13.
Tighten the tube mounting bolts and check the result,
by repeating the steps 7 to 10. If necessary, repeat the steps from step 5.
14.
Before scanning tissues the scan program
“SlideScanner.exe” has to be restarted, otherwise stitching errors may occur.
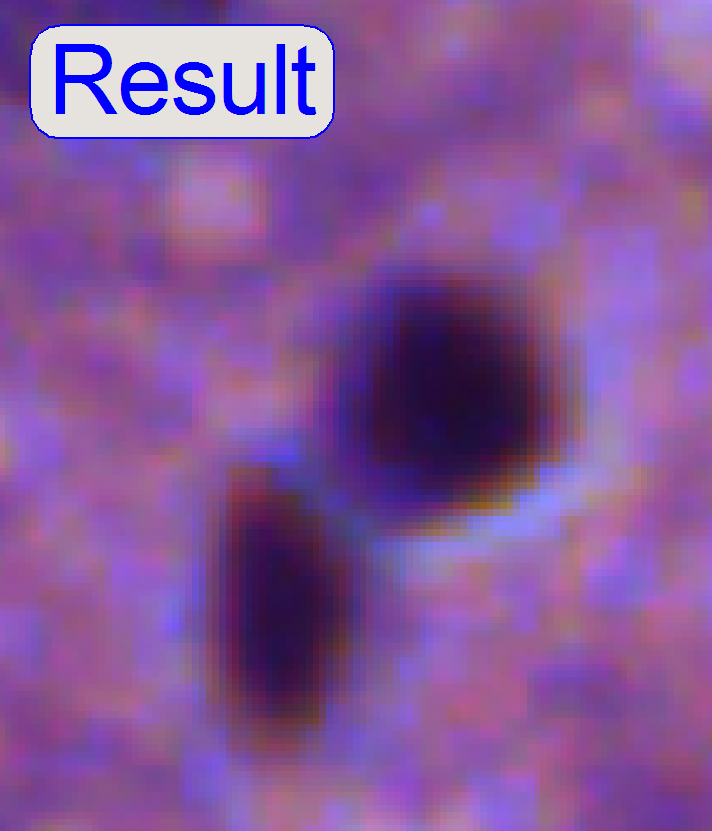 After the
chromatic aberration adjustment was finished, the camera
rotation angle has to be adjusted (again).
After the
chromatic aberration adjustment was finished, the camera
rotation angle has to be adjusted (again).
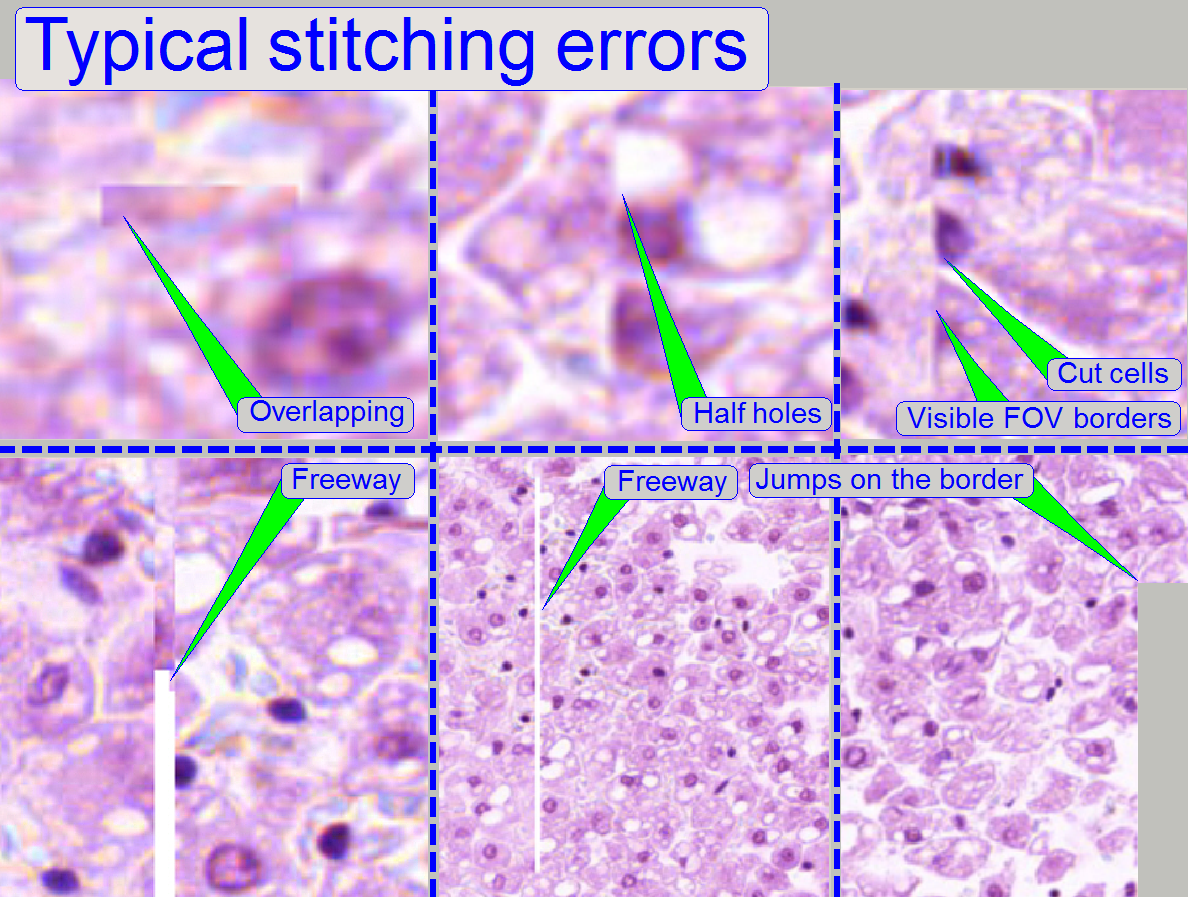 Stitching errors have two main reasons:
Stitching errors have two main reasons:
1.
Improper
adjusted camera rotation angle and
2.
The hysteresis
in Y-direction is too much.
The camera angle becomes important
during stitching. If the angle of the scan camera is out of the limit, the
stitching does not working well, so the FOV’s, seen with the viewer does not
fit to each other. An acceptable camera angle has less then +-0.5 degrees
deviation from zero.
If the camera angle is correct and
stitching errors occur, check the hysteresis in Y-direction.
·
See the next
chapter “Y-
and X-hysteresis” and also “X-Y-stage unit”
Remark
The shown stitching errors existing
always parallel inside of the same scanned tissue, it means, if one occurrence
is found, all others can also be found on different areas of the same scanned
tissue (if the scanned area is large enough).
![]() Stitching; Wikipedia
Stitching; Wikipedia
Adjust the camera rotation angle
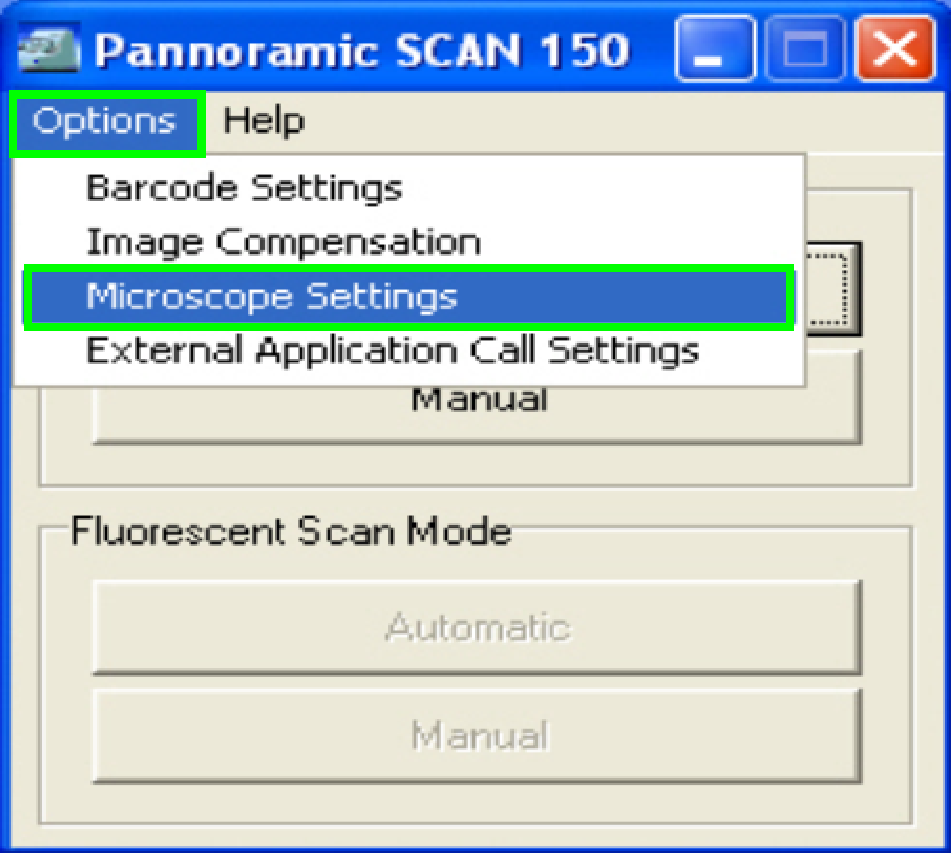 In the
selector menu and ‘Options” start the item “Microscope settings”.
In the
selector menu and ‘Options” start the item “Microscope settings”.
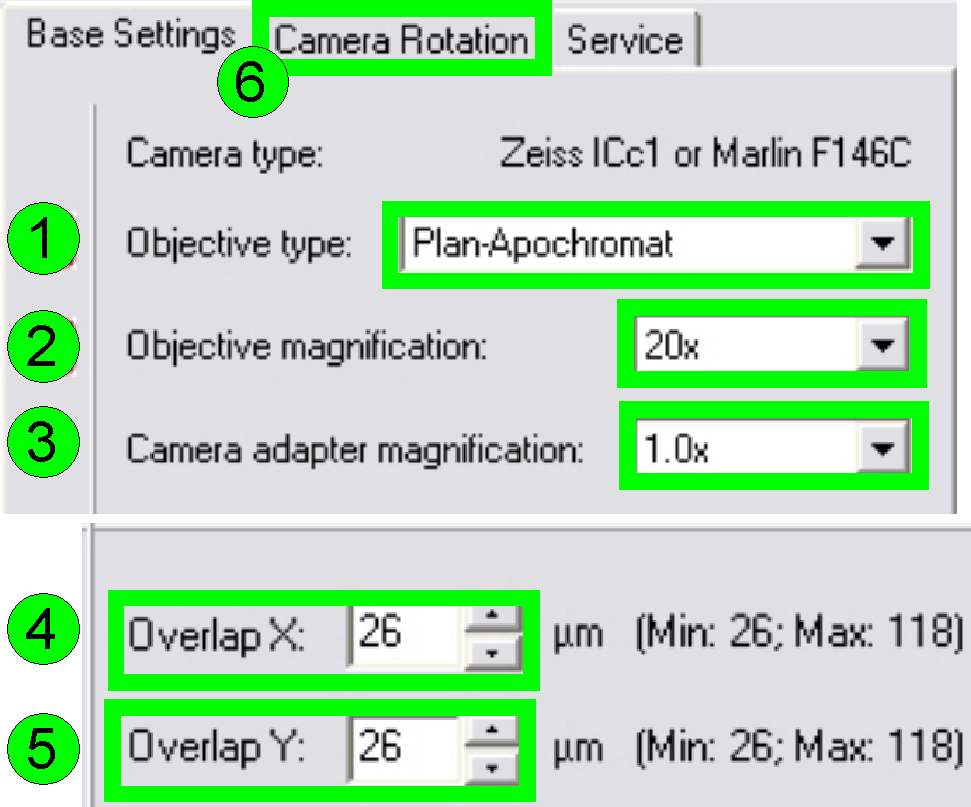 In the
tab “Base settings” set the values for the parameters numbered with (1)-(5) as
these are true for the scanner to be set up; then change to the tab “Camera
rotation” (6).
In the
tab “Base settings” set the values for the parameters numbered with (1)-(5) as
these are true for the scanner to be set up; then change to the tab “Camera
rotation” (6).
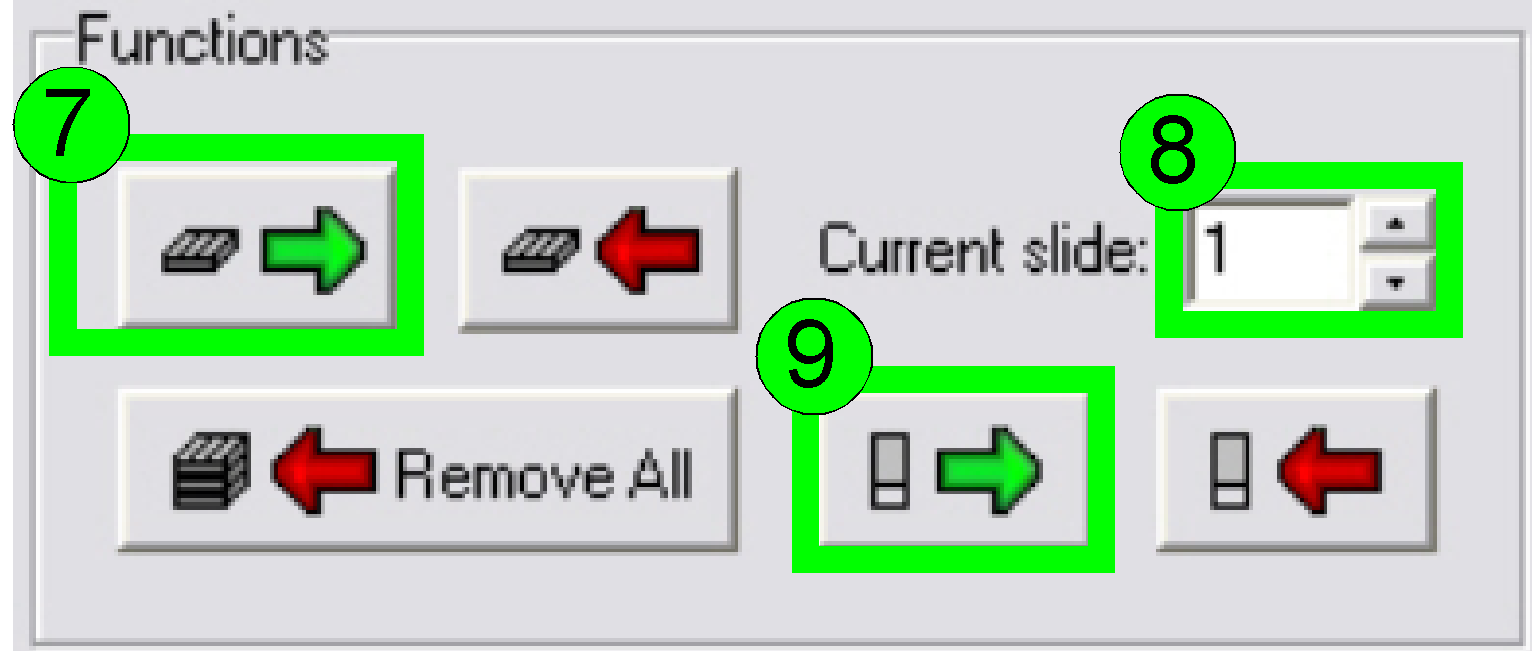
Load a magazine (7), select the desired slide
position (8) and insert the slide (9).
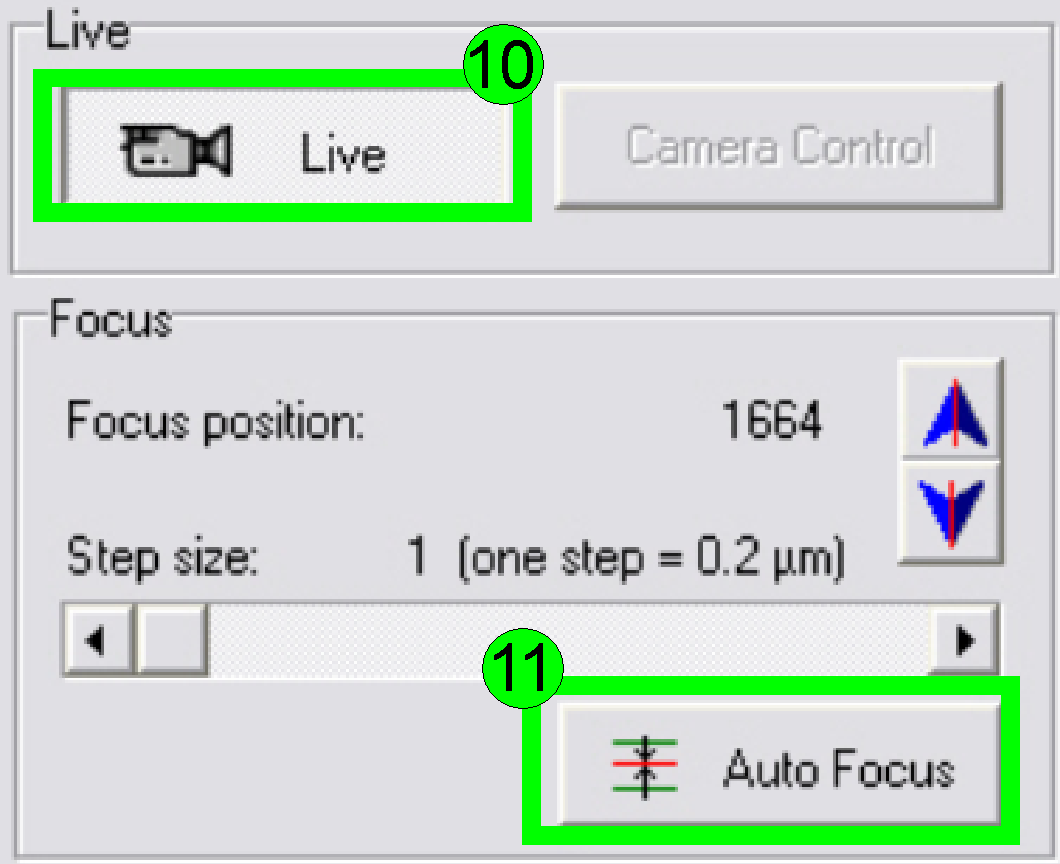
In the preview window find a FOV with tissue, Press
the button “Live view” (10) and “Auto focus” (11). If the focus position is
found, click outside the tissue and inside the cover slip on a “white”
position.
 Set
the “Auto exposure time” and the “White balance” by clicking on the appropriate
icon on the bottom screen border.
Set
the “Auto exposure time” and the “White balance” by clicking on the appropriate
icon on the bottom screen border.
Click inside the tissue and find a well usable FOV
with cells.
Find the focus position (11).
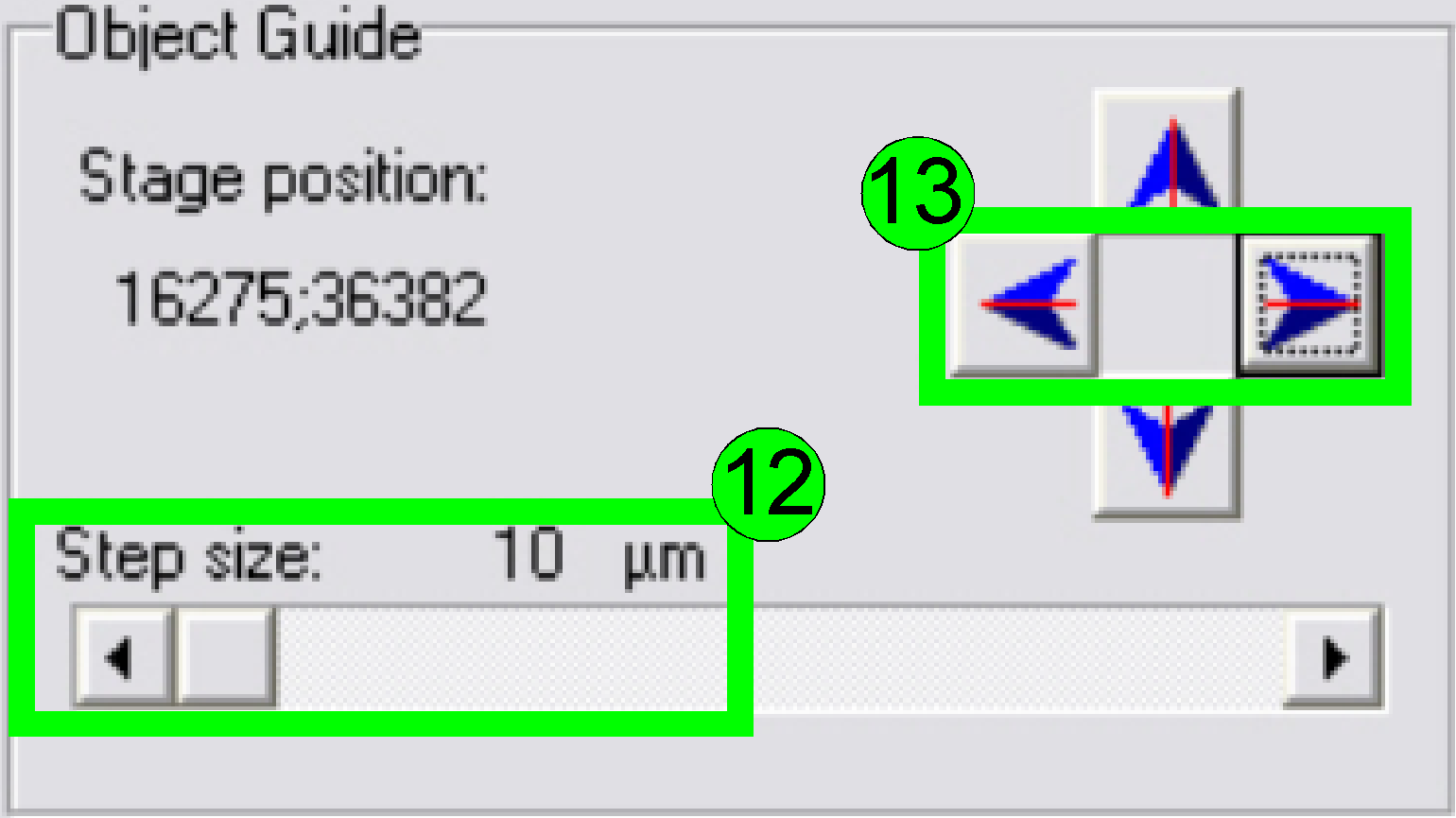 Select
a “Step size” of 10 or 20 µm (12) and move the object guide to the left or
to the right as desired (13) and observe the movement of a cell near to or on
the horizontal red line.
Select
a “Step size” of 10 or 20 µm (12) and move the object guide to the left or
to the right as desired (13) and observe the movement of a cell near to or on
the horizontal red line.
· If the
cell deviates from the red (horizontal) line in the center upward or downward
respectively, correct the camera angle continuously (by moving the camera
adapter on its mounting) until the cell moves on the red line (14) or exact
parallel to it.
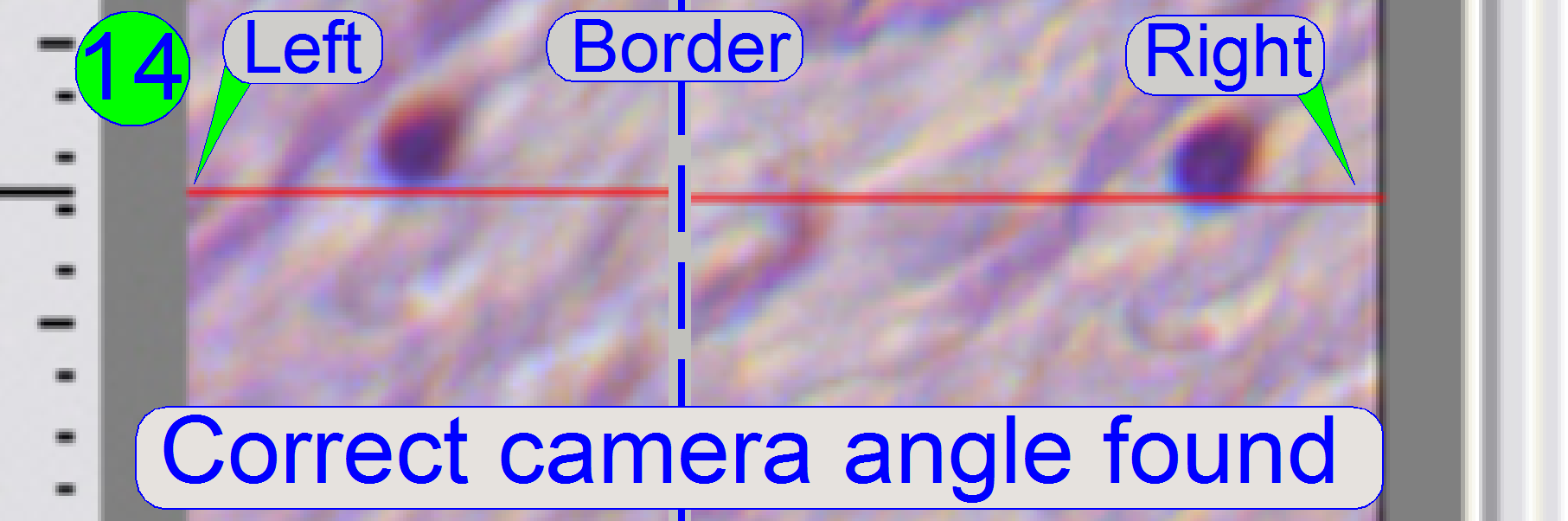 If the
cell moves from the left border to the right border of the screen (or reverse)
nearly on the red line, the camera angle is correct (14).
If the
cell moves from the left border to the right border of the screen (or reverse)
nearly on the red line, the camera angle is correct (14).
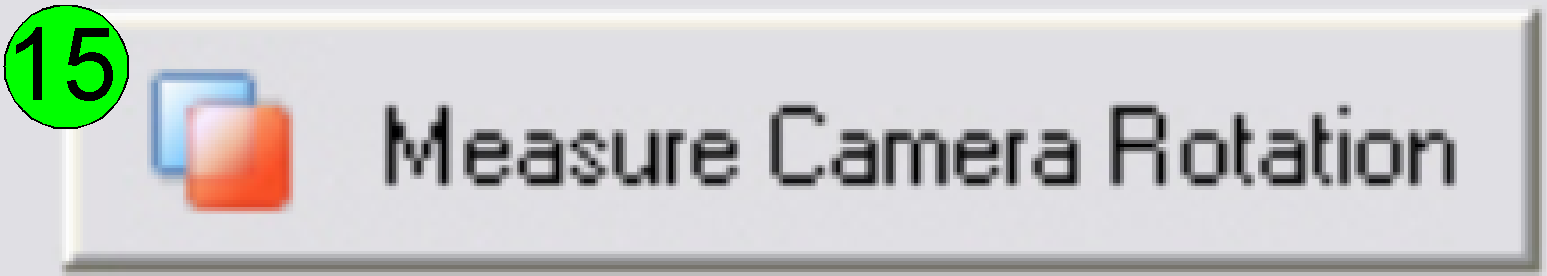 Press
the button “Measure camera rotation” (15).
Press
the button “Measure camera rotation” (15).
 Now the
program arranges two FOVs to each other and shows so graphically the fitting of
the FOVs in the centre of the live view; the numerical value of deviation is
shown in the lower part of the left sided adjustment window.
Now the
program arranges two FOVs to each other and shows so graphically the fitting of
the FOVs in the centre of the live view; the numerical value of deviation is
shown in the lower part of the left sided adjustment window.
· If the
value of the rotation angle is shown in red, the position must be adjusted more
precise (16). Correct the camera position and press the button “Measure camera
rotation” (15) again, until an acceptable angle is found.
· An
acceptable camera rotation angle has less than 0.5degrees deviation from zero.
 If the
rotation angle can be accepted, the angle value is shown in black (17).
If the
rotation angle can be accepted, the angle value is shown in black (17).
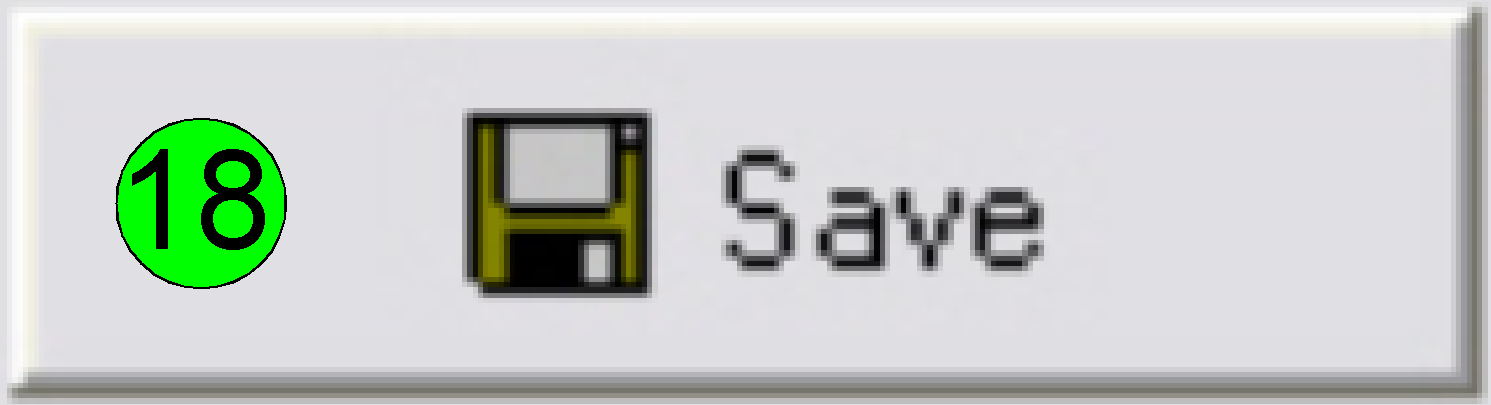
Save the calculated rotation angle to the appropriate file
by pressing “Save” (18); and in the next following dialog answer with “YES” to
save the file.
Leave the menu “Options” by clicking on “Exit”.
Check
the optical path adjustments
As discussed previously, the correct objective and
focus position is important to be able to scan tissues of different thicknesses
in focus.
This fact we are using to determine the correct
objective position.
1. Find at
least three, better are 5 slides with tissue of different thickness and of
different kind.
2. Insert
the (next) slide; check the correct position of the slide in the specimen
holder!
3. Produce
a live view of the tissue, press “Autofocus” and notify the focus position.
4. Repeat
step 3 on 5 different positions of this tissue; the distance of the positions
should be as much as possible.
5. Calculate
the average focus position of this slide and notify it.
6. Repeat
from step 2 until the average focus position of all the selected tissues is
determined.
7. Calculate
the average focus position of all the tissues.
8. If the
average focus position deviates more then 50 steps from the nominal focus
position, calculated with the used slide thickness, the objective position
should be corrected.
9.
If the objective position was modified,
please check the correctness of the condenser position again.
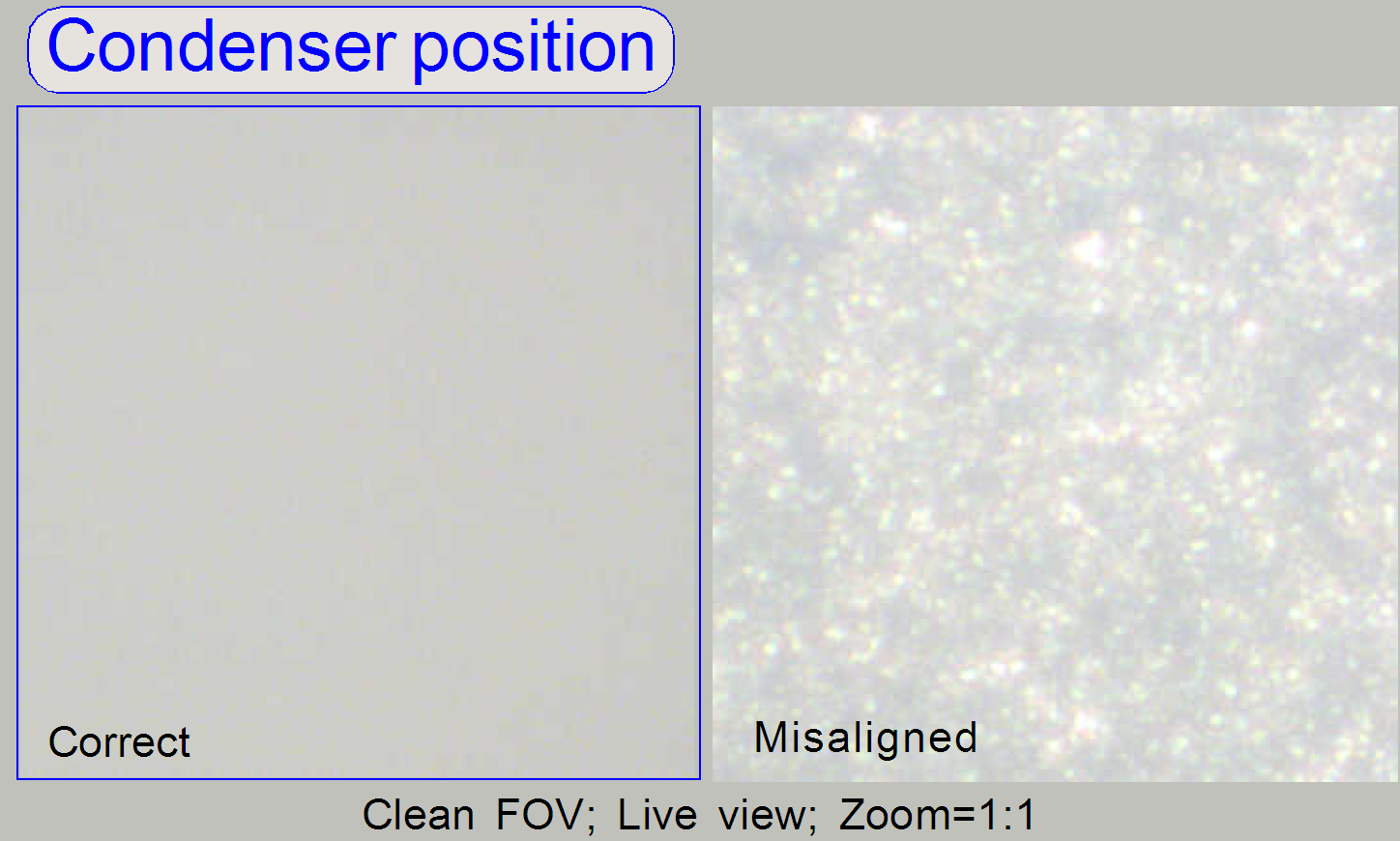 Check the
correct condenser position in the focus positions 1200, 1600 and 2000 steps.
There must not be significant differences.
Check the
correct condenser position in the focus positions 1200, 1600 and 2000 steps.
There must not be significant differences.
· For
best scan results, the clean FOV should be evenly illuminated over the entire
focus range.
· If the
condenser is misaligned, the roughly surface of the diffuser becomes visible!
Remark
“Clean FOV” means a Field of View, seen by the scan
camera without tissue, dust or dirt, between slide and cover slip.
![]() above “Adjust the condenser
position” and “Focus unit”
above “Adjust the condenser
position” and “Focus unit”
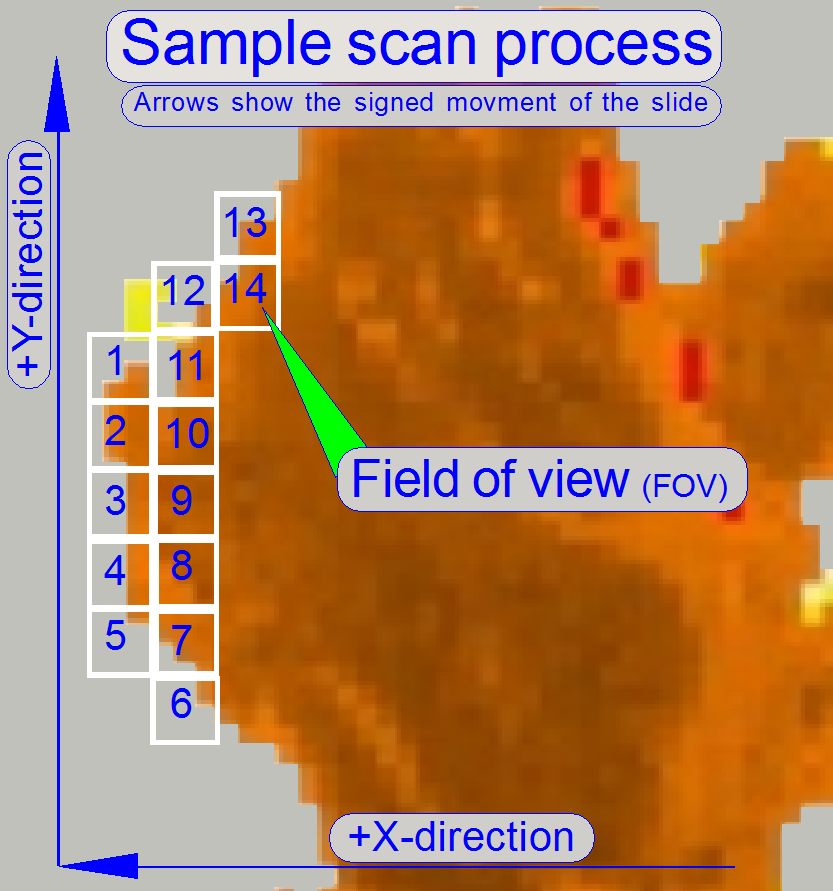
The software
divides the sample to be scanned, seen by the preview camera into fields of
views; the size of the FOV depends on the resolution and the size of the scan
camera’s CCD and the magnification of the camera adapter. Each field of view
contains a small part of the neighbor FOV. In this way, stitching becomes
possible. Because the capturing of the FOV’s is done on a meandering course,
the Y-direction is often changed. If the hysteresis in Y-direction is too much,
stitching will not work correctly; therefore, we have to check the hysteresis
in Y-direction. The maximal allowed hysteresis is
Because the X-direction is never changed during a
sample scan process, the X-hysteresis is not critical and can be some steps
more (max: 8 steps).
· To
reduce the Y-hysteresis, see also “X-Y-stage unit”
and “X- and
Y-carriage drive unit”.
Watch video: “Tissue scan process” (P250)
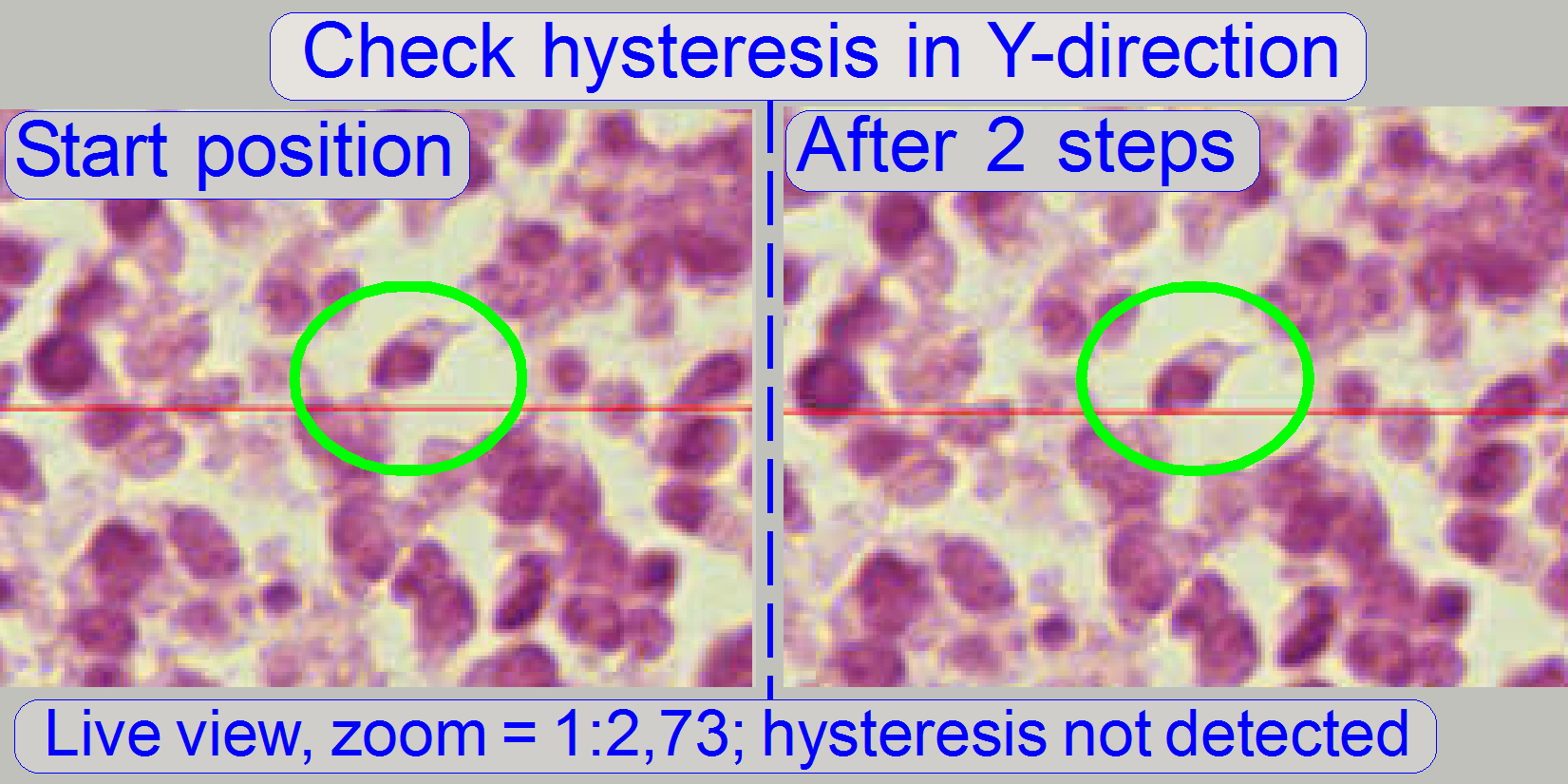
Check
the maximal hysteresis in Y-direction
 Start
the program “SlideScanner.exe” with the service password. In the tab “Focus”
produce a sharp life view.
Start
the program “SlideScanner.exe” with the service password. In the tab “Focus”
produce a sharp life view.
In the tab “Service” select “Microscope control”. In the
part of the X-Y-control select a step size of two steps and go upward, until
the tissue moves.
Now go in opposite direction and count the clicks
until the tissue moves again. If more than 3 clicks are required, the
hysteresis is too much.
The correction of the hysteresis can not be done in
the field.
Scan a tissue and check the chromatic aberration with
the Slide Viewer program.
![]() the chapter above “chromatic aberration”.
the chapter above “chromatic aberration”.
Scan a tissue and check the FOV stitching with the
Slide Viewer program for stitching errors.
![]() “Typical stitching errors” in
the chapter above.
“Typical stitching errors” in
the chapter above.
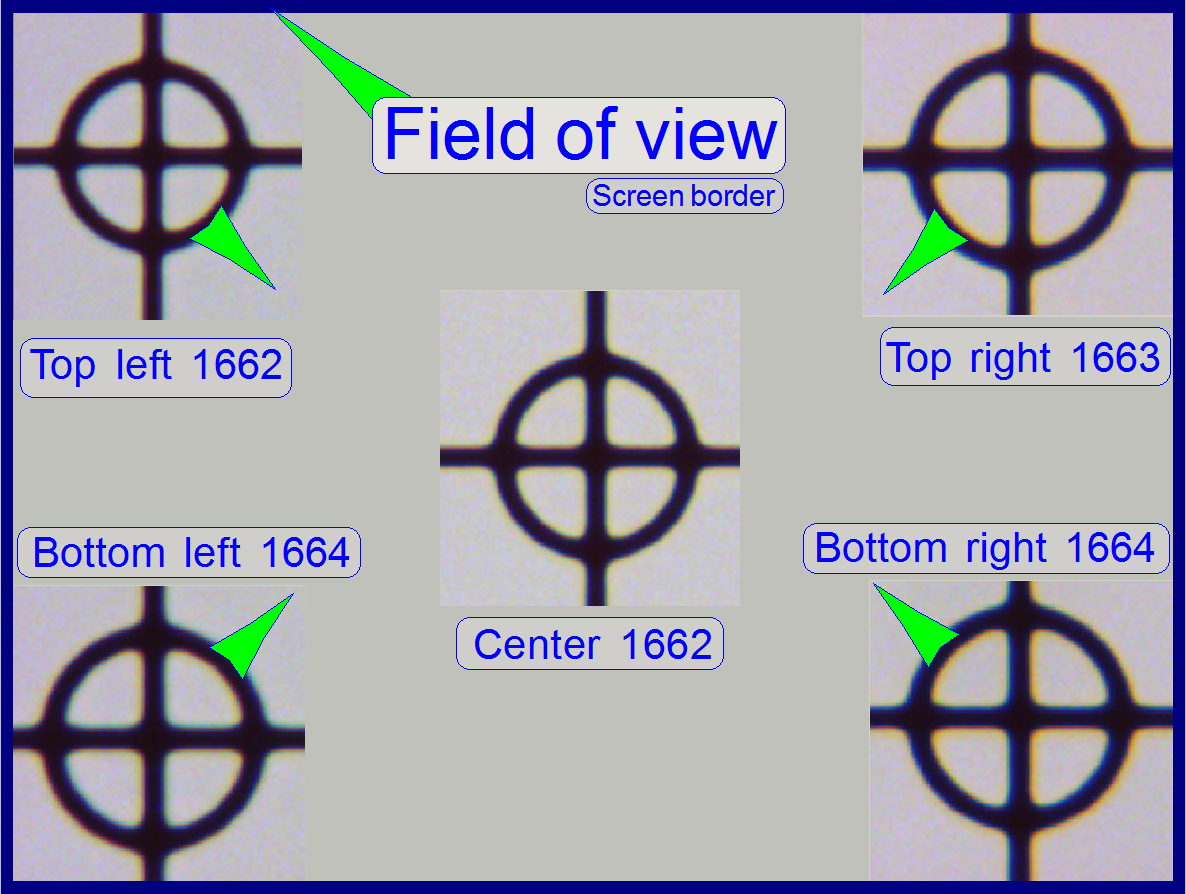
The stage skew check is used to determine the
inclination of the specimen holder and so the inclination of the slide. If the
inclination is too much, parts of the tissue are in focus during other parts of
the same FOV are not in focus.
The Stage skew check should be done:
- If the parallelogram was removed.
- If the parallelogram or the specimen holder was exchanged.
- If the entire X-Y-stage unit was changed.
- If the Focus unit was exchanged.
- If any spare part was changed and this spare part is in connection
with the perpendicularity of the optical axis to the slide.
- If the mounting bolt positions or the adjustment bolts position of
the parallelogram was altered.
To check the inclination angle of the specimen holder,
a series of screen shoots is done of a cell (circle) in the center of the FOV
and in the upper and lower and left and right corners respectively.
There are 7 screenshots taken in each position; 3
before the found auto focus position and 3 screenshots after the auto focus
position. Then find the screenshot of each position where the cell (circle) is
most in focus. If there is a difference, more then 2 focus steps to the found
focus positions, the specimen holder is slanted and has to be adjusted; this
adjustment can not be done in the field; probably the specimen holder or the
parallelogram is deformed.
Important: Always check the proper
position of the slide in the specimen holder first.
In the example on the right the most difference is 2
steps and therefore the inclination of the specimen holder is acceptable.
1.
Start the program SlideScanner.exe with
the service password, insert the slide with circle, produce a live view and
press auto focus.
·
Important: Always check the proper
position of the slide in the specimen holder.
2. Find the
circle and bring it nearly into the center of the live view, press auto focus.
3. Select
the tab “Service” and “Microscope control”.
4. Select
a step rate about 5 or 10 steps for the object guide.
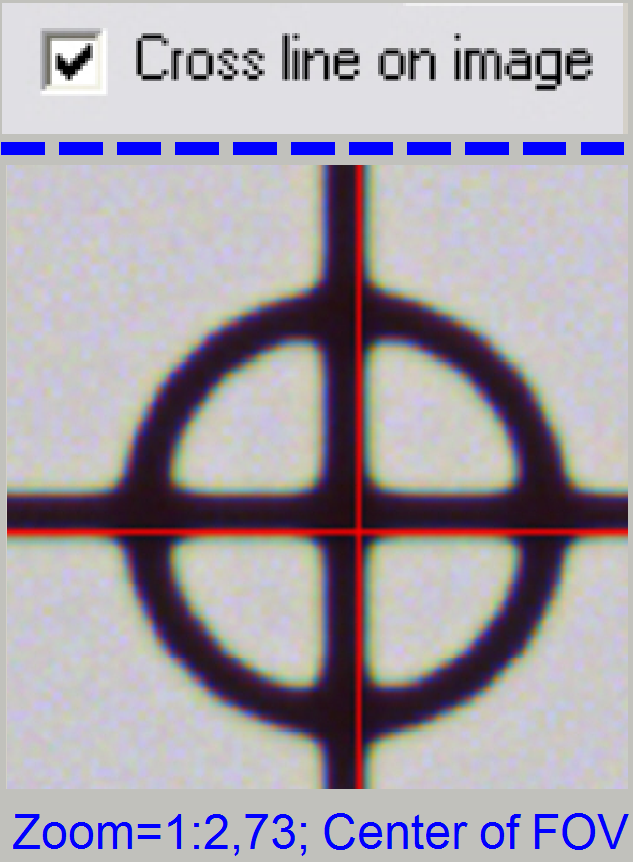
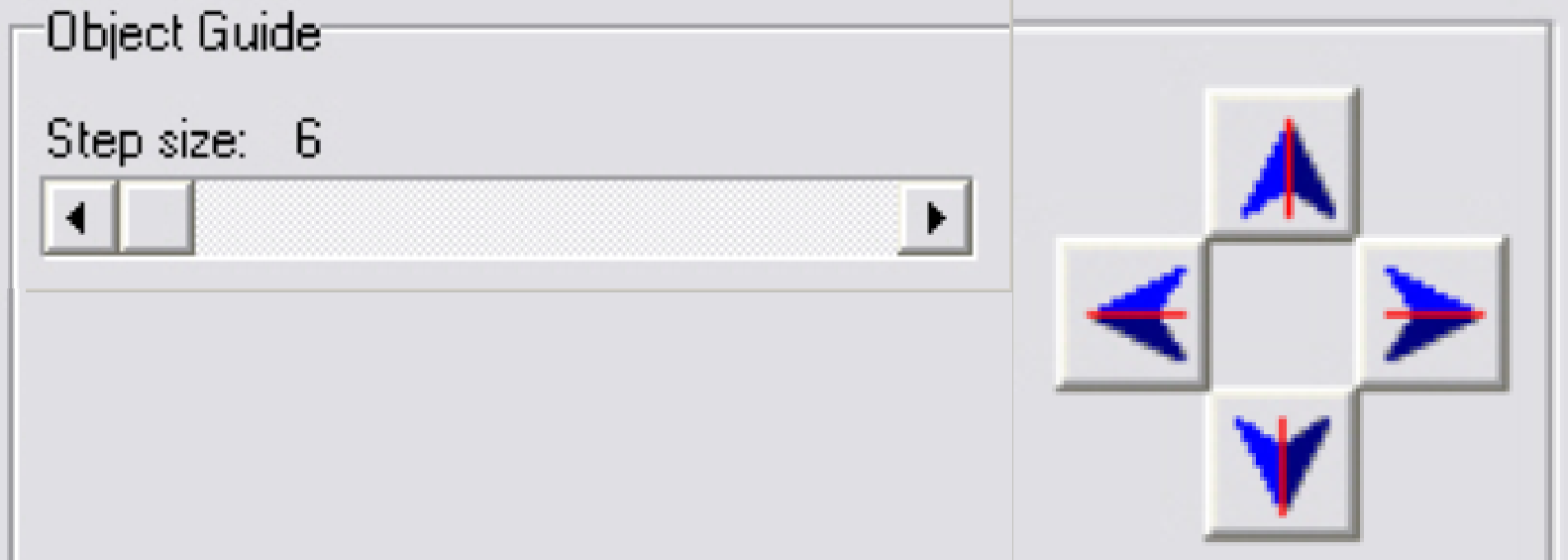 5. Check
the checkbox “Cross line on image” and with the object guide movement buttons
bring the center of the circle to the center of the cross; the circle is now in
the center of the FOV.
5. Check
the checkbox “Cross line on image” and with the object guide movement buttons
bring the center of the circle to the center of the cross; the circle is now in
the center of the FOV.
6. Uncheck
the checkbox “Cross line on image”
7. Zoom
in until a value of 2,73 is reached.
8. Grab
the center of the circle (FOV) into the middle of the screen.
9. Memorize
the auto focus position and go backward with the focus position about 20 steps;
and then go forward to the auto focus position -3 steps with a step size by 1.
This way, the probably hysteresis of the focus unit and other mechanics is
eliminated.
10. Make a screenshot
and create a directory named “Focus stack”, name the file as C (for center) and
the number of the actual focus steps, e.g. “C 1659” if the
memorized focus position was 1662 steps and save the file into the directory
“Focus stack”.
 11. Increment the
focus position by 1, make the next screenshot and save the file.
11. Increment the
focus position by 1, make the next screenshot and save the file.
12. Repeat step 11
until all the 7 screenshots are done.
13. Now move the
circle with the object guide positioning buttons to a corner position, e.g. to
the upper left corner. The corner is found correctly if the circle can not be
grabbed in direction to the center (see also the green arrows in the image
above “The field of view”).
14. Repeat the steps
from step 9 logically until the screenshots are done in all four corners. The
file names should be TL xxxx, BL xxxx, TR xxxx and BR xxxx (for Top Left and so on).
Find the screenshot with the circle most in focus for
each series and notify the file names.
Decide the specimen holder has either to be adjusted
or not as shown in the image above “The field of view”).